Simultaneous Preparation of Abundant Flavonol Triglycosides from Tea Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Uploading Volume on the Preparation of Flavonol Glycoside-Enriched Fraction
2.2. Impact of Elution Method on the Composition of Flavonol Glycoside-Enriched Fraction
2.3. Impact of Flow Rate on the Composition of Flavonol Glycoside-Enriched Fraction
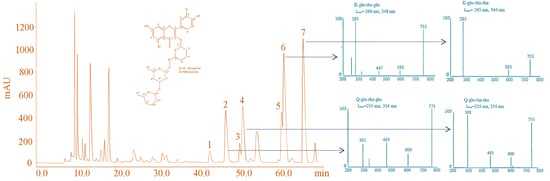
2.4. Isolation and Characterization of Tri-Glycosides
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Tea Extract
3.3. Preparation of Flavonol Glycoside-Enriched Fraction Using Polyamide Column
3.3.1. Polyamide Column Packing
3.3.2. Effect of Uploading Volume
3.3.3. Effect of Different Elution Methods
3.3.4. Effect of Flow Rate
3.4. Preparative HPLC
3.5. UPLC–DAD–MS/MS Analysis of Flavonol Glycosides
3.6. HPLC Analysis
3.7. 1H and 13C Nuclear Magnetic Resonance (NMR) Measurement
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis Teas: A comprehensive review. Compr. Rev. Food Sci. F. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [Green Version]
- Harbowy, M.E.; Balentine, D.A. Tea chemistry. Crit. Rev. Plant. Sci. 1997, 16, 415–480. [Google Scholar] [CrossRef]
- Yang, Z.; Tu, Y.; Baldermann, S.; Dong, F.; Xu, Y.; Watanabe, N. Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. Lwt-Food Sci. Technol. 2009, 42, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.H.; Augustin, M.A. Nano- and micro-particles for delivery of catechins: Physical and biological performance. Crit. Rev. Food Sci. Nutr. 2019, 59, 1563–1579. [Google Scholar] [CrossRef]
- Shi, M.; Wang, Z.S.; Huang, L.Y.; Dong, J.J.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R.; Ye, J.H. Utilization of albumin fraction from defatted rice bran to stabilize and deliver (-)-epigallocatechin gallate. Food Chem. 2020, 311, 125894. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhang, Y.N.; Chen, J.X.; Wang, F.; Du, Q.Z.; Yin, J.F. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Jiang, X.L.; Zhang, S.X.; Dai, X.L.; Liu, Y.J.; Tan, H.R.; Gao, L.P.; Xia, T. Quantification of flavonol glycosides in Camellia sinensis by MRM mode of UPLC-QQQ-MS/MS. J. Chromatogr. B 2016, 1017, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Scharbert, S.; Stark, T. Molecular and gustatory characterisation of the impact taste compounds in black tea infusions. Dev. Food Sci. 2006, 43, 3–8. [Google Scholar]
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J. Agr. Food Chem. 2004, 52, 3498–3508. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Zhou, P.; Wu, H.Y.; Chu, G.X.; Xie, Z.W.; Bao, G.H. Inhibition of α-glucosidase and α-amylase by flavonoid glycosides from Lu’an GuaPian tea: Molecular docking and interaction mechanism. Food Funct. 2018, 9, 4173–4183. [Google Scholar] [CrossRef]
- Browning, A.M.; Walle, U.K.; Walle, T. Flavonoid glycosides inhibit oral cancer cell proliferation—role of cellular uptake and hydrolysis to the aglycones. J. Pharm. Pharmacol. 2005, 57, 1037–1042. [Google Scholar] [CrossRef]
- Xiao, J.B. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Fang, Z.T.; Song, C.J.; Xu, H.R.; Ye, J.H. Dynamic changes in flavonol glycosides during production of green, yellow, white, oolong and black teas from Camellia sinensis L. (cv. Fudingdabaicha). Int. J. Food Sci. Tech. 2019, 54, 490–498. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Nie, Y.; Gao, Y.; Huang, B.; Ye, J.H.; Lu, J.L.; Liang, Y.R. Screening the cultivar and processing factors based on the flavonoid profiles of dry teas using principal component analysis. J. Food Compos. Anal. 2018, 67, 29–37. [Google Scholar] [CrossRef]
- Hilal, Y.; Engelhardt, U.H. A new myricetin-rhamnodiglucoside from Camellia sinensis. Nat. Prod. Res. 2009, 23, 1621–1629. [Google Scholar] [CrossRef]
- Finger, A.; Engelhardt, U.H.; Wray, V. Flavonol triglycosides containning galactose in tea. Phytochemistry 1991, 30, 2057–2060. [Google Scholar] [CrossRef]
- Lakenbrink, C.; Lam, T.M.L.; Engelhardt, U.H.; Wray, V. New flavonol triglycosides from tea (Camellia sinensis). Nat. Prod. Lett. 2000, 14, 233–238. [Google Scholar] [CrossRef]
- Lin, L.Z.; Chen, P.; Harnly, J.M. New phenolic components and chromatographic profiles of green and fermented teas. J. Agr. Food Chem. 2008, 56, 8130–8140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.Y.; Engelhardt, U.H.; Thrane, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef]
- Finger, A.; Engelhardt, U.H.; Wray, V. Flavonol glycosides in tea—kaempferol and quercetin rhamnodiglucosides. J. Sci. Food Agr. 1991, 55, 313–321. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Lin, L.Z.; Harnly, J.M.; Yu, L.L.; Li, Z.W. Tentative identification, quantitation, and principal component analysis of green pu-erh green, and white teas using UPLC/DAD/MS. Food Chem. 2011, 126, 1269–1277. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.J.; Gao, Y.; Liu, Y.; Zheng, X.Q.; Ye, J.H.; Liang, Y.R.; Lu, J.L. Studies on the mechanism of efficient extraction of tea components by aqueous ethanol. Food Chem. 2016, 194, 312–318. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Q-glu-rha-glu, Q-glu-rha-rha, K-glu-rha-glu and K-glu-rha-rha are available from the authors. |




| Fractions | TB | EGC | Caffeine | EC | EGCG | GCG | ECG | TFG |
|---|---|---|---|---|---|---|---|---|
| Method A | ||||||||
| Aqueous | 18.73 ± 0.54 | ND | 254.52 ± 6.37 | ND | ND | ND | ND | ND |
| 60% Methanol | ND | 240.79 ± 12.19 | ND | 35.11 ± 2.39 | ND | ND | ND | 140.13 ± 1.56 |
| 100% Methanol | ND | 287.64 ± 10.49 | ND | 24.35 ± 0.67 | 370.61 ± 7.89 | ND | 162.78 ± 3.10 | 48.11 ± 2.03 |
| Method B | ||||||||
| Aqueous | 19.27 ± 0.09 | ND | 261.08 ± 0.79 | ND | ND | ND | ND | ND |
| 15% Methanol | ND | ND | 3.74 ± 0.20 | ND | ND | ND | ND | ND |
| 30% Methanol | ND | ND | ND | ND | ND | ND | ND | 17.64 ± 2.02 |
| 45% Methanol | ND | 211.88 ± 11.73 | ND | 24.20 ± 0.75 | ND | ND | ND | 103.26 ± 4.31 |
| 70% Methanol | ND | 320.36 ± 5.33 | ND | 61.64 ± 0.97 | 18.93 ± 2.59 | ND | ND | 61.46 ± 2.57 |
| 100% Methanol | ND | ND | ND | ND | 784.26 ± 24.72 | 21.16 ± 1.29 | 246.79 ± 6.26 | 9.17 ± 1.56 |
| Method A | Method B | |||||
|---|---|---|---|---|---|---|
| 60% Methanol | Methanol | 30% Methanol | 45% Methanol | 70% Methanol | Methanol | |
| M-gal | ND | 9.42 ± 0.12 | ND | ND | 4.06 ± 0.73 | 5.11 ± 0.49 |
| M-glu | ND | 7.31 ± 0.02 | ND | ND | 5.23 ± 0.18 | 2.06 ± 0.27 |
| Q-glu-rha-gal | 4.82 ± 0.06 | 0.71 ± 0.50 | ND | 3.06 ± 0.09 | 2.29 ± 0.11 | ND |
| Q-glu-rha-glu | 20.76 ± 0.04 | 1.16 ± 0.06 | 2.76 ± 0.39 | 16.47 ± 0.28 | 3.4 ± 0.24 | ND |
| Q-gal-rha-rha | 7.86 ± 0.05 | 2.44 ± 0.10 | ND | 4.39 ± 0.14 | 6.43 ± 0.24 | 0.15 ± 0.21 |
| Q-glu-rha-rha | 13.27 ± 0.02 | 0.84 ± 0.04 | 0.88 ± 0.16 | 10.86 ± 0.22 | 2.87 ± 0.12 | ND |
| Q-glu-rha | 3.60 ± 0.10 | 0.91 ± 0.04 | ND | 2.04 ± 0.06 | 2.21 ± 0.15 | ND |
| K-gal-rha-glu | 3.42 ± 0.07 | ND | 0.84 ± 0.08 | 2.14 ± 0.11 | 0.38 ± 0.05 | ND |
| Q-gal | ND | 2.74 ± 0.07 | ND | ND | 1.77 ± 0.07 | 0.63 ± 0.46 |
| Q-glu | ND | 6.93 ± 0.04 | ND | ND | 6.13 ± 0.02 | 1.22 ± 0.13 |
| K-gal-rha-rha | 7.30 ± 0.02 | 0.25 ± 0.18 | 1.18 ± 0.12 | 5.54 ± 0.17 | 1.05 ± 0.08 | ND |
| K-glu-rha-glu | 37.76 ± 0.13 | 1.61 ± 0.28 | 7.41 ± 0.76 | 28.56 ± 0.76 | 4.59 ± 0.24 | ND |
| K-glu-rha-rha | 38.00 ± 0.15 | 2.11 ± 0.10 | 4.56 ± 0.51 | 28.85 ± 2.42 | 6.41 ± 0.10 | ND |
| K-gal | ND | 1.10 ± 0.04 | ND | ND | 1.38 ± 0.08 | ND |
| K-glu-rha | 2.09 ± 0.03 | 0.14 ± 0.20 | ND | 1.35 ± 0.07 | 1.31 ± 0.03 | ND |
| K-glu | 1.25 ± 0.88 | 10.46 ± 0.23 | ND | ND | 11.96 ± 0.16 | ND |
| Q-gal-rha-glu | Q-glu-rha-glu | Q-gal-rha-rha | Q-glu-rha-rha | Q-glu-rha | K-gal-rha-glu | K-gal-rha-rha | K-glu-rha-glu | K-glu-rha-rha | K-glu-rha | TFG | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 mL/min | 3.56 ± 0.11 a | 19.27 ± 0.77 a | 4.38 ± 0.17 a | 12.46 ± 0.38 a | 2.19 ± 0.18 a | 2.42 ± 0.12 a | 6.62 ± 0.28 a | 33.84 ± 1.62 a | 35.38 ± 1.32 a | 1.57 ± 0.04 a | 121.69 ± 4.99 a |
| 5 mL/min | 3.06 ± 0.09 b | 16.47 ± 0.28 b | 4.39 ± 0.14 a | 10.86 ± 0.22 b | 2.04 ± 0.06 a | 2.14 ± 0.11 b | 5.54 ± 0.17 b | 28.56 ± 0.76 b | 28.85 ± 2.42 b | 1.35 ± 0.07 b | 103.26 ± 4.31 b |
| 8 mL/min | 3.08 ± 0.19 b | 15.66 ± 0.2 c | 4.25 ± 0.53 a | 10.12 ± 0.35 b | 2.15 ± 0.37 a | 2.38 ± 0.28 ab | 5.38 ± 0.06 b | 27.86 ± 0.41 b | 28.9 ± 0.58 b | 1.34 ± 0.15 b | 101.11 ± 3.12 b |
| Compounds | Abbreviation | Content |
|---|---|---|
| Theobromine | 1.19 ± 0.03 | |
| Theophylline | 0.05 ± 0.01 | |
| Caffeine | 18.09 ± 0.50 | |
| (−)-gallocatechin | GC | 2.97 ± 0.08 |
| (−)-Epigallocatechin | EGC | 26.62 ± 1.29 |
| (+)-catechin | C | 1.49 ± 0.04 |
| (−)-Epicatechin | EC | 5.01 ± 0.14 |
| (−)-Epigallocatechin gallate | EGCG | 74.94 ± 2.07 |
| (−)-Gallocatechin gallate | GCG | 3.75 ± 0.10 |
| (−)-Epicatechin gallate | ECG | 19.64 ± 0.54 |
| (+)-catechin gallate | CG | 0.48 ± 0.01 |
| Myricetin-3-O-rhamnosyl-glucoside | M-glu-rha | 0.11 ± 0.01 |
| Myricetin-3-O-galactoside | M-gal | 0.44 ± 0.05 |
| Myricetin-3-O-glucoside | M-glu | 0.36 ± 0.04 |
| Quercetin-3-O-glucosyl-rhamnosyl-galactoside | Q-gal-rha-glu | 0.32 ± 0.01 |
| Quercetin-3-O-glucosyl-rhamnosyl-glucoside | Q-glu-rha-glu | 1.15 ± 0.04 |
| Quercetin-3-O-rhamnosyl-rhamnosyl-galactoside | Q-gal-rha-rha | 0.54 ± 0.02 |
| Quercetin-3-O-rhamnosyl-rhamnosyl-glucoside | Q-glu-rha-rha | 0.73 ± 0.02 |
| Quercetin-3-O-rhamnosyl-glucoside | Q-glu-rha | 0.28 ± 0.01 |
| Kaempferol-3-O-glucosyl-rhamnosyl-galactoside | K-gal-rha-glu | 0.16 ± 0.01 |
| Quercetin-3-O-galactoside | Q-gal | 0.13 ± 0.02 |
| Quercetin-3-O-glucoside | Q-glu | 0.33 ± 0.04 |
| Kaempferol-O-rhamnosyl-rhamnosyl-galactoside | K-gal-rha-rha | 0.41 ± 0.01 |
| Kaempferol-3-O-glucosyl-rhamnosyl-glucoside | K-glu-rha-glu | 2.06 ± 0.05 |
| Kaempferol-O-rhamnosyl-rhamnosyl-glucoside | K-glu-rha-rha | 2.13 ± 0.02 |
| Kaempferol-3-O-galactoside | K-gal | 0.08 ± 0.01 |
| Kaempferol-3-O-rhamnosyl-glucoside | K-glu-rha | 0.14 ± 0.01 |
| Kaempferol-3-O-glucoside | K-glu | 0.57 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.-T.; Lv, Y.-Q.; Song, C.-J.; Jin, J.; Lu, J.-L.; Xu, H.-R.; Ye, J.-H. Simultaneous Preparation of Abundant Flavonol Triglycosides from Tea Leaves. Molecules 2020, 25, 5140. https://doi.org/10.3390/molecules25215140
Fang Z-T, Lv Y-Q, Song C-J, Jin J, Lu J-L, Xu H-R, Ye J-H. Simultaneous Preparation of Abundant Flavonol Triglycosides from Tea Leaves. Molecules. 2020; 25(21):5140. https://doi.org/10.3390/molecules25215140
Chicago/Turabian StyleFang, Zhou-Tao, Yi-Qing Lv, Chu-Jun Song, Jing Jin, Jian-Liang Lu, Hai-Rong Xu, and Jian-Hui Ye. 2020. "Simultaneous Preparation of Abundant Flavonol Triglycosides from Tea Leaves" Molecules 25, no. 21: 5140. https://doi.org/10.3390/molecules25215140
APA StyleFang, Z. -T., Lv, Y. -Q., Song, C. -J., Jin, J., Lu, J. -L., Xu, H. -R., & Ye, J. -H. (2020). Simultaneous Preparation of Abundant Flavonol Triglycosides from Tea Leaves. Molecules, 25(21), 5140. https://doi.org/10.3390/molecules25215140