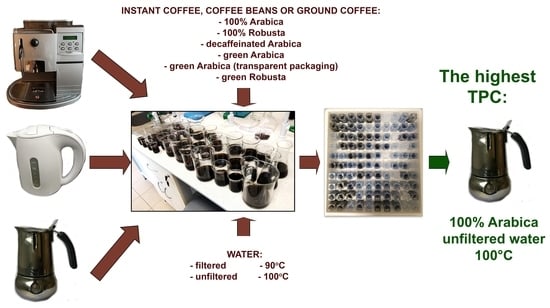
Impact of Brewing Methods on Total Phenolic Content (TPC) in Various Types of Coffee
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic Content in 100% Arabica Coffee
2.2. Total Phenolic Content in 100% Robusta Coffee
2.3. Total Phenolic Content in Arabica Decaffeinated Coffee
2.4. Total Phenolic Content in Green Arabica Coffee
2.5. Total Phenolic Content in Green Arabica Coffee in Transparent Packaging
2.6. Total Phenolic Content in Green Robusta Coffee
2.7. Daily Consumption of Polyphenols with Coffee
2.8. Principal Components Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Coffee Materials
4.2. Coffee Brewing
4.3. Total Phenolic Content Analysis
4.4. Estimation of Daily Intake of Phenolic Compounds
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Coffee Organization: Historical Data on the Global Coffee Trade. Available online: http://www.ico.org/new_historical.asp (accessed on 7 March 2020).
- Clifford, M.N. Coffee: Botany, Biochemistry and Production of Beans and Beverage, 1st ed.; The AVI Publishing Company, INC.: Westport, CT, USA, 1985. [Google Scholar]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Coffee, Tea, Cocoa. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Żukiewicz-Sobczak, W.; Krasowska, E.; Sobczak, P.; Horoch, A.; Wojtyła, A.; Piątek, J. Wpływ spożycia kawy na organizm człowieka [Effect of coffee consumption on the human body]. Medycyna Ogólna Nauki o Zdrowiu [Gen. Med. Health Sci.] 2012, 18, 71–76. (In Polish) [Google Scholar]
- Bae, J.H.; Park, J.H.; Im, S.S.; Song, D.K. Coffee and health. Integr. Med. Res. 2014, 3, 189–191. [Google Scholar] [CrossRef] [Green Version]
- Cano-Marquina, A.; Tarín, J.J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieber, K. The impact of coffee on health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Herden, L.; Weissert, R. The effect of coffee and caffeine consumption on patients with multiple sclerosis-related fatigue. Nutrients 2020, 12, 2262. [Google Scholar] [CrossRef]
- Rodas, L.; Riera-Sampol, A.; Aguilo, A.; Martínez, S.; Tauler, P. Effects of habitual caffeine intake, physical activity levels, and sedentary behavior on the inflammatory status in a healthy population. Nutrients 2020, 12, 2325. [Google Scholar] [CrossRef]
- Vacek, J.; Ulrichová, J.; Klejdus, B.; Simánek, V. Analytical methods and strategies in the study of plant polyphenolics in clinical samples. Anal. Methods 2010, 2, 604–613. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Smilin Bell Aseervatham, G.; Sivasudha, T.; Jeyadevi, R.; Arul Ananth, D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans—An overview. Environ. Sci. Pollut. Res. Int. 2013, 20, 4356–4369. [Google Scholar] [CrossRef] [PubMed]
- Moeenfard, M.; Rocha, L.; Alves, A. Quantification of caffeoylquinic acids in coffee brews by HPLC-DAD. J. Anal. Methods Chem. 2014, 2014, 965353. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Colomban, S.; Azinheira, H.G.; Guerra-Guimarães, L.; Do Céu Silva, M.; Navarini, L.; Resmini, M. Dietary antioxidants in coffee leaves: Impact of botanical origin and maturity on chlorogenic acids and xanthones. Antioxidants 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Chłopicka, J.; Niedziela, A.; Bartoń, H. Aktywność antyoksydacyjna i całkowita zawartość polifenoli w naparach kawy w zależności od rodzaju kawy i sposobu jej przygotowania [Antioxidant activity and total polyphenol content in coffee infusions depending on the type of coffee and the method of its preparation]. Bromat Chem. Toksykol. [Bromat Toxicol. Chem.] 2015, 48, 278–282. (In Polish) [Google Scholar]
- Severini, C.; Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A. Roasting conditions, grinding level and brewing method highly affect the healthy benefits of a coffee cup. Int. J. Clin. Nutr Diet. 2018, 4, JCND-127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priftis, A.; Stagos, D.; Konstantinopoulos, K.; Tsitsimpikou, C.; Spandidos, D.A.; Tsatsakis, A.M.; Tzatzarakis, M.N.; Kouretas, D. Comparison of antioxidant activity between green and roasted coffee beans using molecular methods. Mol. Med. Rep. 2015, 12, 7293–7302. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Bacăniciu, I.; Pop, A.; Tofenă, M.; Muste, S.; Feier, D. Evaluation of biofunctional compounds content from brewed coffee. Bulletin UASVM Food Science and Technology 2014, 7. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Merecz, A.; Marusińska, A.; Karwowski, B.T. The content of biologically active substances and antioxidant activity in coffee depending on brewing method. Pol. J. Natur. Sci. 2018, 33, 267–284. [Google Scholar]
- Wolska, J.; Janda, K.; Jakubczyk, K.; Szymkowiak, M.; Chlubek, D.; Gutowska, I. Levels of antioxidant activity and fluoride content in coffee infusions of Arabica, Robusta and green coffee beans in according to their brewing methods. Biol. Trace Elem. Res. 2017, 179, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Extraction of coffee antioxidants: Impact of brewing time and method. Food Res. 2012, 48, 57–64. [Google Scholar] [CrossRef]
- Michalak-Majewska, M. Analiza zawartości szczawianów w popularnych naparach herbat i kaw [Analysis of oxalate content in popular tea and coffee infusions]. Bromat Chem. Toksykol. [Bromat Toxicol. Chem.] 2013, 46, 74–79. (In Polish) [Google Scholar]
- Sperkowska, B.; Bazylak, G. Analiza zawartości szczawianów w naparach herbat i kaw dostępnych na polskim rynku [Analysis of oxalate content in infusions of teas and coffees available on the Polish market]. Nauka. Przyroda. Technol. [Sci. Nat. Technol.] 2010, 4, 1–13. (In Polish) [Google Scholar]
- De Marco, I.; Riemma, S.; Iannone, R. Supercritical carbon dioxide decaffeination process: A life cycle assessment study. Chem. Eng. Trans. 2017, 57, 1699–1704. [Google Scholar] [CrossRef]
- Pietsch, A. Decaffeination: Process and quality. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 225–243. [Google Scholar]
- Rozporządzenie Ministra Rolnictwa i Rozwoju Wsi z dnia 23 grudnia 2014 r. w sprawie znakowania poszczególnych rodzajów środków spożywczych [Regulation of the Minister of Agriculture and Rural Development of 23 December 2014 on Labeling Foodstuffs]; Ministry of Agriculture and Rural Development, Dz.U.: Warsaw, Poland, 2015; p. 29. (In Polish)
- Mills, C.E.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. The effect of processing on chlorogenic acid content of commercially available coffee. Food Chem. 2013, 141, 3335–3340. [Google Scholar] [CrossRef]
- Hall, S.; Yuen, J.W.; Grant, G.D. Bioactive constituents in caffeinated and decaffeinated coffee and their effect on the risk of depression—A comparative constituent analysis study. Beverages 2018, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Sarriá, B.; Martínez-López, S.; Mateos, R.; Bravo-Clemente, L. Long-term consumption of a green/roasted coffee blend positively affects glucose metabolism and insulin resistance in humans. Food Res. 2016, 89, 1023–1028. [Google Scholar] [CrossRef]
- Sarriá, B.; Martínez-López, S.; Sierra-Cinos, J.L.; García-Diz, L.; Mateos, R.; Bravo-Clemente, L. Regularly consuming a green/roasted coffee blend reduces the risk of metabolic syndrome. Eur. J. Nutr. 2018, 57, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.; Ashiharad, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef]
- Tfouni, S.A.; Serrate, C.S.; Carreiro, L.B.; Camargo, M.C.; Teles, C.R.; Cipolli, K.M.; Furlani, R.P. Effect of roasting on chlorogenic acids, caffeine and polycyclic aromatic hydrocarbons levels in two Coffea cultivars: Coffea arabica cv. Catuaí Amarelo IAC-62 and Coffea canephora cv. Apoatã IAC-2258. Int. J. Food Sci. 2012, 47, 406–415. [Google Scholar] [CrossRef]
- Moon, J.K.; Yoo, H.S.; Shibamoto, T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: Correlation with coffee acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, K.; Wołosiak, R. Wpływ prażenia kawy na wybrane parametry jej jakości [Influence of coffee roasting on selected parameters of its quality]. Aparatura Badawcza i Dydaktyczna [Res. Teach. Appar.] 2014, 19, 77–83. (In Polish) [Google Scholar]
- Bobková, A.; Hudáček, M.; Jakabová, S.; Belej, Ľ.; Capcarová, M.; Čurlej, J.; Bobko, M.; Árvay, J.; Jakab, I.; Čapla, J.; et al. The effect of roasting on the total polyphenols and antioxidant activity of coffee. J. Environ. Sci. Health B 2020, 55, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Acrylamide carcinogenicity. J. Agric. Food Chem. 2008, 56, 5984–5988. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, P.; Lie, T.; Li, J.; Hutz, J.R.; Li, K.; Shi, F. Reproductive toxicity of acrylamide-treated male rats. Reprod. Toxicol. 2010, 29, 225–230. [Google Scholar] [CrossRef]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a potential functional food ingredients. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT-Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Morales, F.J.; Somoza, V.; Fogliano, V. Physiological relevance of dietary melanoidins. Amino Acids 2012, 42, 1097–1109. [Google Scholar] [CrossRef]
- Trang, V.T.; Takeuchi, H.; Kudo, H.; Aoki, A.; Katsuno, S.; Shimamura, T. Antimicrobial activity of aminoreductone against Helicobacter pylori. J. Agric. Food Chem. 2009, 57, 11343–11348. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; de la Cueva, S.P. Antimicrobial activity of coffee melanoidins—A study of their metal-chelating properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, V.G.; Tureck, C.; Locateli, G.; Peralta, R.M.; Koehnlein, E.A. Estimate of consumption of phenolic compounds by Brazilian population. Rev. Nutr. Campinas 2015, 28, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Koch, W.; Baj, T.; Kukula-Koch, W.; Marzec, Z. Dietary intake of specific phenolic compounds and their effect on the antioxidant activity of daily food rations. Open Chem. 2015, 13, 869–876. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |









| Type of Coffee | n | Average ± SD | Med. | Min.–Max. | Quartile Lower–Upper | IQR |
|---|---|---|---|---|---|---|
| 100% Arabica | 54 | 221.8 ± 212 | 145.8 | 50.4–683.2 | 55.5–371.3 | 315.8 |
| 100% Robusta | 54 | 226.9 ± 200 | 171.6 | 53.4–711.2 | 62.8–347.1 | 284.3 |
| decaffeinated Arabica | 54 | 166.9 ± 134 | 150.0 | 38.8–430.4 | 45.6–266.4 | 220.8 |
| green Arabica | 21 | 106.5 ± 104 | 27.4 | 16.3–329.6 | 21.8–215.8 | 194.0 |
| green Arabica (transparent packaging) | 21 | 142.2 ± 135 | 39.7 | 15.6–378.2 | 31.2–271.0 | 239.8 |
| green Robusta | 21 | 129.0 ± 117 | 59.8 | 20.7–375.8 | 29.1–230.4 | 201.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olechno, E.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. Impact of Brewing Methods on Total Phenolic Content (TPC) in Various Types of Coffee. Molecules 2020, 25, 5274. https://doi.org/10.3390/molecules25225274
Olechno E, Puścion-Jakubik A, Markiewicz-Żukowska R, Socha K. Impact of Brewing Methods on Total Phenolic Content (TPC) in Various Types of Coffee. Molecules. 2020; 25(22):5274. https://doi.org/10.3390/molecules25225274
Chicago/Turabian StyleOlechno, Ewa, Anna Puścion-Jakubik, Renata Markiewicz-Żukowska, and Katarzyna Socha. 2020. "Impact of Brewing Methods on Total Phenolic Content (TPC) in Various Types of Coffee" Molecules 25, no. 22: 5274. https://doi.org/10.3390/molecules25225274
APA StyleOlechno, E., Puścion-Jakubik, A., Markiewicz-Żukowska, R., & Socha, K. (2020). Impact of Brewing Methods on Total Phenolic Content (TPC) in Various Types of Coffee. Molecules, 25(22), 5274. https://doi.org/10.3390/molecules25225274