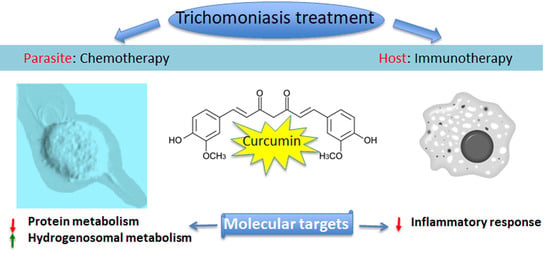
Molecular Targets Implicated in the Antiparasitic and Anti-Inflammatory Activity of the Phytochemical Curcumin in Trichomoniasis
Abstract
:1. Introduction
2. Results
2.1. In Vitro Antiparasitic Effect of Curcumin
2.2. Influence of Curcumin on Pyruvate-Ferredoxine Oxidoreductase (PfoD) Gene Expression and the Hydrogenosomal Membrane Potential (ΔΨm)
2.3. Impact of Curcumin on Parasite Proteolytic Activity
2.4. Influence of Curcumin on the Pro-Inflammatory Processes Induced by Lipopolysaccharide (LPS) and T. vaginalis Proteinases in Immune Cells
2.5. Effects of Curcumin on Other Mediators of Inflammation in Macrophages Stimulated with Lipopolysaccharide (LPS) and Proteinases of T. vaginalis
3. Discussion
4. Materials and Methods
4.1. Parasites
4.2. Culture of Murine Macrophages
4.3. Antiparasitic Activity
4.4. Hydrogenosomal Membrane Potential Assay
4.5. Proteinase Purification
4.6. SDS-PAGE-Gelatin Assay
4.7. Gelatin-FITC Proteolytic Activity
4.8. Assay of Nitrite Production
4.9. Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-qPCR)
4.10. Chemiluminiscent Enzyme-Linked ImmunoSorbent Assay (ELISA)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schwebke, J.R.; Burgess, D. Trichomoniasis. Clin. Microbiol. Rev. 2004, 17, 794–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes, C.B.; Frasson, A.P.; Tasca, T. Trichomoniasis are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microb. Cell (Graz, Austria) 2016, 3, 404–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo, R.; Cárdenas-Guerra, R.E.; Figueroa-Angulo, E.E.; Puente-Rivera, J.; Zamudio-Prieto, O.; Ortega-López, J. Trichomonas vaginalis Cysteine Proteinases: Iron Response in gene expression and proteolytic activity. BioMed Res. Int. 2015, 2015, 946787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, D.; Alderete, J.F. Analysis of human immunoglobulin-degrading cysteine proteinases of Trichomonas vaginalis. Infect Immun. 1995, 63, 3388–3395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Sánchez, M.E.; Ávila-González, L.; Becerril-García, C.; Fattel-Facenda, L.V.; Ortega-López, J.; Arroyo, R. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb. Pathog. 2000, 28, 193–202. [Google Scholar] [CrossRef]
- Hernández-Gutiérrez, R.; Ávila-González, L.; Ortega-López, J.; Cruz-Talonia, F.; Gómez-Gutierrez, G.; Arroyo, R. Trichomonas vaginalis: Characterization of a 39-kDa cysteine proteinase found in patient vaginal secretions. Exp. Parasitol. 2004, 107, 125–135. [Google Scholar] [CrossRef]
- Hernández, H.M.; Sariego, I.; Álvarez, A.B.; Marcet, R.; Vancol, E.; Álvarez, A.; Figueredo, M.; Sarracent, J. Trichomonas vaginalis 62 kDa proteinase as a possible virulence factor. Parasitol. Res. 2011, 108, 241–245. [Google Scholar] [CrossRef]
- Ma, L.; Meng, Q.; Cheng, W.; Sung, Y.; Tang, P.; Hu, S.; Yu, J. Involvement of the GP63 protease in infection of Trichomonas vaginalis. Parasitol. Res. 2011, 109, 71–79. [Google Scholar] [CrossRef]
- Mercer, F.; Johnson, P.J. Trichomonas vaginalis: Pathogenesis, symbiont interations, and host cell immune response. Trends Parasitol. 2018, 34, 683–693. [Google Scholar] [CrossRef]
- Cárdenas-Guerra, R.E.; Arroyo, R.; Rosa de Andrade, I.; Benchimol, M.; Ortega-López, J. The iron-induced cysteine proteinase TvCP4 plays a key role in Trichomonas vaginalis haemolysis. Microbes Infect. 2013, 15, 958–968. [Google Scholar] [CrossRef]
- Rendón-Gandarilla, F.J.; Ramón-Luing, L.D.L.; Ortega-López, J.; Rosa de Andrade, I.; Benchimol, M.; Arroyo, R. The TvLEGU-1, a legumain-like cysteine proteinase, plays a key role in Trichomonas vaginalis cytoadherence. Biomed. Res. Int. 2013, 2013, 561979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalia, N.; Singh, J.; Kaur, M. Immunopathology of Recurrent Vulvovaginal Infections: New Aspects and Research Directions. Front. Immunol. 2019, 10, 2034. [Google Scholar] [CrossRef] [PubMed]
- Quon, D.V.; d’Oliveira, C.E.; Johnson, P.J. Reduced transcription of the ferredoxin gene in metronidazole-resistant Trichomonas vaginalis. Proc. Natl. Acad. Sci. USA 1992, 89, 4402–4406. [Google Scholar] [CrossRef] [Green Version]
- Samarawickrema, N.A.; Brown, D.M.; Upcroft, J.A.; Thammapalerd, N.; Upcroft, P. Involvement of superoxide dismutase and pyruvate:ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. J. Antimicrob. Chemother. 1997, 40, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An update on metabolism, structure-cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulda, J. Trichomonads, hydrogenosomes and drug resistance. Int. J. Parasitol. 1999, 29, 199–212. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, A.; Silva, B.M.; Palmeira-de-Oliveira, R.; Martinez-de-Oliveira, J.; Salgueiro, L. Are plant extracts a potential therapeutic approach for genital infections? Curr. Med. Chem. 2013, 20, 2914–2928. [Google Scholar] [CrossRef]
- Vieira, P.D.B.; Giordani, R.B.; Macedo, A.J.; Tasca, T. Natural and synthetic compound anti-Trichomonas vaginalis: An update review. Parasitol. Res. 2015, 114, 1249–1261. [Google Scholar] [CrossRef]
- Mallo, N.; Lamas, J.; Leiro, J.M. Hydrogenosome metabolism is the key target for antiparasitic activity of resveratrol against Trichomonas vaginalis. Antimicrob. Agents Chemother. 2013, 57, 2476–2484. [Google Scholar] [CrossRef] [Green Version]
- Wachter, B.; Syrowatka, M.; Obwaller, A.; Walochnik, J. In vitro efficacy of curcumin on Trichomonas vaginalis. Wien. Klin. Wochenschr. 2014, 126, S32–S36. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153, Erratum in: Altern. Med. Rev. 2009, 14, 277. [Google Scholar] [PubMed]
- Nagajyothi, F.; Zhao, D.; Weiss, L.M.; Tanowitz, H.B. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol. Res. 2012, 110, 2491–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.T.; Yao, Q.Y.; Xu, B.L.; Wang, J.Y.; Zhou, C.H.; Zhang, S.C. Protective effects of curcumin against hepatic fibrosis induced by carbon tetrachloride: Modulation of high-mobility group box 1, Toll-like receptor 4 and 2 expression. Food Chem. Toxicol. 2012, 50, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef] [PubMed]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 150. [Google Scholar] [CrossRef]
- Gorrell, T.E. Effect of culture medium iron content on the biochemical composition and metabolism of Trichomonas vaginalis. J. Bacteriol. 1985, 161, 1228–1230. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Song, H.O.; Choi, I.H.; Park, S.J.; Ryu, J.S. Hydrogenosomal activity of Trichomonas vaginalis cultivated under different iron conditions. Korean J. Parasitol. 2006, 44, 373–378. [Google Scholar] [CrossRef]
- Aoyagi, S.; Archer, T.K. Differential glucocorticoid receptor-mediated transcription mechanisms. J. Biol. Chem. 2011, 286, 4610–4619. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Dang, T.; Blind, R.D.; Wang, Z.; Cavasotto, C.N.; Hittelman, A.B.; Rogatsky, I.; Logan, S.K.; Garabedian, M.J. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol. Endocrinol. 2008, 22, 1754–1766. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Henrotin, Y.; Biesalski, H.K.; Shakibaei, M. Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health. Int. J. Mol. Sci. 2012, 13, 4202–4232. [Google Scholar] [CrossRef]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS), Scientific Opinion on the reevaluation of curcumin (E 100) as a food additive. EFSA J. 2010, 8, 1679–1725. [Google Scholar] [CrossRef]
- Smutná, T.; Gonçalves, V.L.; Saraiva, L.M.; Tachezy, J.; Teixeira, M.; Hrdy, I. Flavodiiron protein from Trichomonas vaginalis hydrogenosomes: The terminal oxygen reductase. Eukaryot. Cell 2009, 8, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, U.; Costello, C.E.; Hayes, G.R.; Beach, D.H.; Gilbert, R.O.; Lucas, J.J.; Singh, B.N. Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J. Biol. Chem. 2005, 280, 23853–23860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neale, K.A.; Alderete, J.F. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect. Immun. 1990, 58, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Cobo, E.R.; Reed, S.L.; Corbeil, L.B. Effect of vinyl sulfone inhibitors of cysteine proteinases on Tritrichomonas foetus infection. Int. J. Antimicrob. Agents 2012, 39, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Hirt, R.P. Trichomonas vaginalis virulence factors: An integrative overview. Sex. Transm. Infect. 2013, 89, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Vazeille, E.; Slimani, L.; Claustre, A.; Magne, H.; Labas, R.; Béchet, D.; Taillandier, D.; Dardevet, D.; Astruc, T.; Attaix, D.; et al. Curcumin treatment prevents increased proteasome and apoptosome activities in rat skeletal muscle during reloading and improves subsequent recovery. J. Nutr. Biochem. 2012, 23, 245–251. [Google Scholar] [CrossRef]
- Priyanka, A.; Anusree, S.S.; Nisha, V.M.; Raghu, K.G. Curcumin improves hypoxia induced dysfunctions in 3T3-L1 adipocytes by protecting mitochondria and down regulating inflammation. Biofactors 2014, 40, 513–523. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Park, G.C.; Ryu, J.S.; Min, D.Y. The role of nitric oxide as an effector of macrophage-mediated cytotoxicity against Trichomonas vaginalis. Korean J. Parasitol. 1997, 35, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gradoni, L.; Ascenzi, P. Nitric oxide and anti-protozoan chemotherapy. Parassitologia 2004, 46, 101–103. [Google Scholar] [PubMed]
- Trujillo, J.; Granados-Castro, L.F.; Zazueta, C.; Andérica-Romero, A.C.; Chirino, Y.I.; Pedraza-Chaverrí, J. Mitochondria as a target in the therapeutic properties of curcumin. Archiv Pharm. (Weinh.) 2014, 347, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.; Arranz, J.A.; Fraiz, N.; Sanmartín, M.L.; Quezada, E.; Orallo, F. Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling. Int. Immunopharmacol. 2005, 5, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory effects of curcumin in microglial cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teiten, M.H.; Reuter, S.; Schmucker, S.; Dicato, M.; Diederich, M. Induction of heat shock response by curcumin in human leukemia cells. Cancer Lett. 2009, 279, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A. NFkappaB, heat shock proteins, HSF-1, and inflammation. Cardiovasc. Res. 2006, 6, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.Q.; Zhang, Y.; Xiang, J.J.; Xiong, C.L. Protective effect of curcumin against liver warm ischemia/reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J. Gastroenterol. 2007, 13, 1953–1961. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Heikkila, J.J. Curcumin-induced inhibition of proteasomal activity, enhanced HSP accumulation and the acquisition of thermotolerance in Xenopus laevis A6 cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 566–576. [Google Scholar] [CrossRef]
- Xia, C.; Cai, Y.; Li, S.; Yang, J.; Xiao, G. Curcumin increases HSP70 expression in primary rat cortical neuronal apoptosis induced by gp120 V3 loop peptide. Neurochem. Res. 2015, 40, 1996–2005. [Google Scholar] [CrossRef]
- Diamond, L.S. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 1957, 43, 488–490. [Google Scholar] [CrossRef] [PubMed]
- León-Rodríguez, L.; Luzardo-Álvarez, A.; Blanco-Méndez, J.; Lamas, J.; Leiro, J. Biodegradable microparticles covalently linked to surface antigens of the scuticociliate parasite P. dicentrarchi promote innate immune responses in vitro. Fish Shellfish Immunol. 2013, 34, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Paramá, A.; Iglesias, R.; Álvarez, M.F.; Leiro, J.; Ubeira, F.M.; Sanmartín, M.L. Cysteine proteinase activities in the fish pathogen Philasterides dicentrarchi (Ciliophora: Scuticociliatida). Parasitology 2004, 128, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Piazzon, C.; Lamas, J.; Leiro, J.M. Role of scuticociliate proteinases in infection success in turbot, Psetta maxima (L.). Parasite Immunol. 2011, 33, 535–544. [Google Scholar] [CrossRef]
- Griess, P. Bemerkungen zu der abhandlung der HH. Weselsky und Benedikt “Ueber einige azoverbindungen”. Chem. Ber. 1879, 12, 426–428. [Google Scholar] [CrossRef] [Green Version]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2- Delta Delta Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Piazzon, C.; Lamas, J.; Castro, R.; Budiño, B.; Cabaleiro, S.; Sanmartín, M.L.; Leiro, J. Antigenic and cross-protection studies on two turbot scuticociliate isolates. Fish Shellfish Immunol. 2008, 25, 417–424. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds curcumin are available from the authors. |






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallo, N.; Lamas, J.; Sueiro, R.A.; Leiro, J.M. Molecular Targets Implicated in the Antiparasitic and Anti-Inflammatory Activity of the Phytochemical Curcumin in Trichomoniasis. Molecules 2020, 25, 5321. https://doi.org/10.3390/molecules25225321
Mallo N, Lamas J, Sueiro RA, Leiro JM. Molecular Targets Implicated in the Antiparasitic and Anti-Inflammatory Activity of the Phytochemical Curcumin in Trichomoniasis. Molecules. 2020; 25(22):5321. https://doi.org/10.3390/molecules25225321
Chicago/Turabian StyleMallo, Natalia, Jesús Lamas, Rosa Ana Sueiro, and José Manuel Leiro. 2020. "Molecular Targets Implicated in the Antiparasitic and Anti-Inflammatory Activity of the Phytochemical Curcumin in Trichomoniasis" Molecules 25, no. 22: 5321. https://doi.org/10.3390/molecules25225321
APA StyleMallo, N., Lamas, J., Sueiro, R. A., & Leiro, J. M. (2020). Molecular Targets Implicated in the Antiparasitic and Anti-Inflammatory Activity of the Phytochemical Curcumin in Trichomoniasis. Molecules, 25(22), 5321. https://doi.org/10.3390/molecules25225321