The Effect of Filtration on Physical and Chemical Properties of Osmo-Dehydrated Material
Abstract
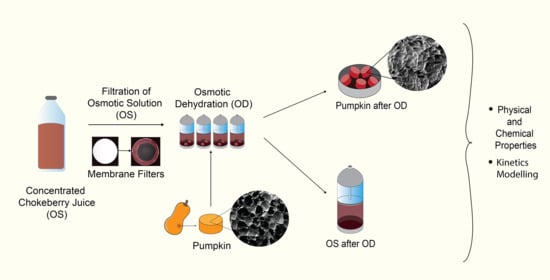
:1. Introduction
2. Results and Discussion
2.1. Physical and Chemical Properties of Osmotic Solutions Before the Osmotic Dehydration
2.2. Osmotic Dehydration
2.3. Properties of Pumpkin Cylinders after Osmotic Dehydration
2.4. Properties of Osmotic Solution (OS) after Osmotic Dehydration
3. Materials and Methods
3.1. Materials
3.2. Osmotic Solution
3.3. Osmotic Dehydration
3.4. Physical and Chemical Analysis
3.4.1. Moisture Content (MC)
3.4.2. Water Activity (aw)
3.4.3. Concentration of Osmotic Solution
3.4.4. Density of Osmotic Solution
3.4.5. Viscosity of Osmotic Solution
3.4.6. Preparation of the Samples for Chemical Analysis
3.4.7. Determination of Total Phenolic Content and Antioxidant Capacity (TEAC ABTS and FRAP Methods)
3.5. SEM Imaging
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pieters, B.; Assis, F.R.; Morais, R.M.S.C.; De Morais, A.M.M.B. Quality of ‘Royal Gala’ cut apple during osmotic dehydration. Braz. J. Food Technol. 2020, 23, 2019059. [Google Scholar] [CrossRef]
- Lenart, A.; Lewicki, P. Osmotic Dehydration of Fruits and Vegetables. In Handbook of Industrial Drying; CRC Press: London, UK, 2006. [Google Scholar]
- Sahoo, P.; Sharma, A. Modelling of water loss and solute uptake during osmotic drying of carrots using weibull distribution approach. Int. Food Res. J. 2018, 25, 270–274. [Google Scholar]
- Samborska, K.; Eliasson, L.; Marzec, A.; Kowalska, J.; Piotrowski, D.; Lenart, A.; Kowalska, H. The effect of adding berry fruit juice concentrates and by-product extract to sugar solution on osmotic dehydration and sensory properties of apples. J. Food Sci. Technol. 2019, 56, 1927–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, J.A.G.; Ruiz-López, I.I.; Martínez-Sánchez, C.E.; Rodríguez-Miranda, J.; Carmona-Garcia, R.; Torruco-Uco, J.G.; Ochoa-Martínez, L.A.; Herman-Lara, E. Effect of osmotic dehydration on the physical and chemical properties of Mexican ginger (Zingiber officinalevar. Grand Cayman). CyTA J. Food 2015, 14, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Mundada, M.; Hathan, B.S.; Maske, S. Mass Transfer Kinetics during Osmotic Dehydration of Pomegranate Arils. J. Food Sci. 2010, 76, E31–E39. [Google Scholar] [CrossRef]
- Li, H.; Ramaswamy, H.S. Osmotic Dehydration: Dynamics of Equilibrium and Pseudo-Equilibrium Kinetics. Int. J. Food Prop. 2010, 13, 234–250. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M. Osmotic dehydration of foods: Mass transfer and modeling aspects. Food Rev. Int. 2002, 18, 305–335. [Google Scholar] [CrossRef]
- Phisut, N. Factors affecting mass transfer during osmotic dehydration of fruits. Int. Food Res. J. 2012, 19, 7–18. [Google Scholar]
- Cichowska-Bogusz, J.; Żubernik, J.; Czyzewski, J.; Kowalska, H.; Witrowa-Rajchert, D. Efficiency of Osmotic Dehydration of Apples in Polyols Solutions. Molecules 2018, 23, 446. [Google Scholar] [CrossRef] [Green Version]
- Panagiotou, N.; Karathanos, V.; Maroulis, Z. Effect of osmotic agent on osmotic dehydration of fruits. Dry. Technol. 1999, 17, 175–189. [Google Scholar] [CrossRef]
- Lech, K.; Michalska, A.; Wojdyło, A.; Nowicka, P.; Figiel, A. The influence of physical properties of selected plant materials on the process of osmotic dehydration. LWT Food Sci. Technol. 2018, 91, 588–594. [Google Scholar] [CrossRef]
- Saurel, R.; Rios, G.; Guilbert, S.; Raoult-Wack, A.-L. Mass transfer phenomena during osmotic dehydration of apple I. Fresh plant tissue. Int. J. Food Sci. Technol. 2007, 29, 531–542. [Google Scholar] [CrossRef]
- Lech, K.; Michalska, A.; Wojdyło, A.; Nowicka, P.; Figiel, A. The Influence of the Osmotic Dehydration Process on Physicochemical Properties of Osmotic Solution. Molecules 2017, 22, 2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goula, A.M.; Kokolaki, M.; Daftsiou, E. Use of ultrasound for osmotic dehydration. The case of potatoes. Food Bioprod. Process. 2017, 105, 157–170. [Google Scholar] [CrossRef]
- Çağlayan, D.; Mazi, I.B.; Mazi, I.B. Effects of ultrasound-assisted osmotic dehydration as a pretreatment and finish drying methods on the quality of pumpkin slices. J. Food Process. Preserv. 2018, 42, e13679. [Google Scholar] [CrossRef]
- Lech, K.; Figiel, A.; Wojdyło, A.; Korzeniowska, M.; Serowik, M.; Szarycz, M. Drying Kinetics and Bioactivity of Beetroot Slices Pretreated in Concentrated Chokeberry Juice and Dried with Vacuum Microwaves. Dry. Technol. 2015, 33, 1644–1653. [Google Scholar] [CrossRef]
- Lech, K.; Figiel, A.; Michalska, A.; Wojdyło, A.; Nowicka, P. The Effect of Selected Fruit Juice Concentrates Used as Osmotic Agents on the Drying Kinetics and Chemical Properties of Vacuum-Microwave Drying of Pumpkin. J. Food Qual. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Antonyuk, S.; Heinrich, S.; Deen, N.; Kuipers, J. Influence of liquid layers on energy absorption during particle impact. Particuology 2009, 7, 245–259. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Gasiorowski, K.; Szyba, K.; Brokos, B.; Kołaczyńska, B.; Jankowiak-Włodarczyk, M.; Oszmiański, J. Antimutagenic activity of anthocyanins isolated from Aronia melanocarpa fruits. Cancer Lett. 1997, 119, 37–46. [Google Scholar] [CrossRef]
- Chua, L.Y.W.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Lech, K.; Chua, B.L. Characterisation of the Convective Hot-Air Drying and Vacuum Microwave Drying of Cassia alata: Antioxidant Activity, Essential Oil Volatile Composition and Quality Studies. Molecules 2019, 24, 1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bchir, B.; Besbes, S.; Karoui, R.; Paquot, M.; Attia, H.; Blecker, C. Osmotic Dehydration Kinetics of Pomegranate Seeds Using Date Juice as an Immersion Solution Base. Food Bioprocess Technol. 2010, 5, 999–1009. [Google Scholar] [CrossRef]
- Kucner, A.; Klewicki, R.; Sójka, M. The Influence of Selected Osmotic Dehydration and Pretreatment Parameters on Dry Matter and Polyphenol Content in Highbush Blueberry (Vaccinium corymbosum L.) Fruits. Food Bioprocess Technol. 2013, 6, 2031–2047. [Google Scholar] [CrossRef] [Green Version]
- Cano-Lamadrid, M.; Lech, K.; Michalska, A.; Wasilewska, M.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Influence of osmotic dehydration pre-treatment and combined drying method on physico-chemical and sensory properties of pomegranate arils, cultivar Mollar de Elche. Food Chem. 2017, 232, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Raoult-Wack, A.-L.; Guilbert, S.; Le Maguer, M.; Rios, G. Simultaneous water and solute transport in shrinking media—Part 2. Dry. Technol. 1991, 9, 589–612. [Google Scholar] [CrossRef]
- Masztalerz, K.; Lech, K.; Wojdyło, A.; Nowicka, P.; Michalska-Ciechanowska, A.; Figiel, A. The impact of the osmotic dehydration process and its parameters on the mass transfer and quality of dried apples. Dry. Technol. 2020, 1–13. [Google Scholar] [CrossRef]
- Kowalska, H.; Lenart, A.; Leszczyk, D. The effect of blanching and freezing on osmotic dehydration of pumpkin. J. Food Eng. 2008, 86, 30–38. [Google Scholar] [CrossRef]
- Cichowska-Bogusz, J.; Witrowa-Rajchert, D.; Stasiak-Różańska, L.; Figiel, A. Ultrasound-Assisted Osmotic Dehydration of Apples in Polyols and Dihydroxyacetone (DHA) Solutions. Molecules 2019, 24, 3429. [Google Scholar] [CrossRef] [Green Version]
- Cichowska-Bogusz, J.; Figiel, A.; Stasiak-Różańska, L.; Witrowa-Rajchert, D. Modeling of Osmotic Dehydration of Apples in Sugar Alcohols and Dihydroxyacetone (DHA) Solutions. Foods 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialik, M.; Wiktor, A.; Latocha, P.; Gondek, E. Mass Transfer in Osmotic Dehydration of Kiwiberry: Experimental and Mathematical Modelling Studies. Molecules 2018, 23, 1236. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, C.; Guo, Y.; An, K.; Ding, S.; Wang, Z. Mass transfer evaluation of ultrasonic osmotic dehydration of cherry tomatoes in sucrose and salt solutions. Int. J. Food Sci. Technol. 2012, 47, 954–960. [Google Scholar] [CrossRef]
- Almeida, J.A.; Mussi, L.P.; Oliveira, D.B.; Pereira, N.R. Effect of Temperature and Sucrose Concentration on the Retention of Polyphenol Compounds and Antioxidant Activity of Osmotically Dehydrated Bananas. J. Food Process. Preserv. 2014, 39, 1061–1069. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Lech, K.; Figiel, A. Chemical Composition, Antioxidant Capacity, and Sensory Quality of Dried Sour Cherry Fruits pre-Dehydrated in Fruit Concentrates. Food Bioprocess Technol. 2015, 8, 2076–2095. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Peleg, M. An Empirical Model for the Description of Moisture Sorption Curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoidesL.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| Osmotic Solution | Water Activity aw (-) | Density kg·m−3 | Viscosity mPa·s | TPC mg GA·100 g−1 dm | TEAC ABTS mmol Trx·100 g−1 dm | FRAP mmol Trx·g−1 dm |
|---|---|---|---|---|---|---|
| NF | 0.9424 ± 0.0008 b | 1200.42 ± 13.2 a | 2.85 ± 0.08 c | 3501.3 ± 102.9 b | 49.7 ± 1.22 f | 32.9 ± 0.81 b |
| 8 μm | 0.9438 ± 0.0001 a,b,c | 1189.16 ± 17.84 a | 2.77 ± 0.08 b,c | 3257.4 ± 56.7 a | 38.09 ± 0.94 a | 32.9 ± 0.81 b |
| 5 μm | 0.9444 ± 0.0005 a,c | 1181.25 ± 9.45 a | 2.74 ± 0.07 a,b,c | 3282.9 ± 73.4 a | 38.89 ± 0.96 a,b | 32.51 ± 0.8 a,b |
| 3 μm | 0.9446 ± 0.0001 a,c | 1190.24 ± 20.23 a | 2.59 ± 0.07 a,b | 3293.4 ± 9.2 a | 44.99 ± 1.11 d | 30.73 ± 0.76 a,b |
| 1.2 μm | 0.9428 ± 0.0008 a,b | 1191.53 ± 14.3 a | 2.61 ± 0.08 a,b | 3361.9 ± 87.5 a,b | 33.99 ± 0.84 e | 31 ± 0.76 a,b |
| 0.8 μm | 0.9434 ± 0.0006 a,b | 1187.24 ± 15.43 a | 2.62 ± 0.05 a,b | 2681.2 ± 8 c | 43.29 ± 1.06 c,d | 35.26 ± 0.87 c |
| 0.45 μm | 0.9429 ± 0.0002 a,b | 1188.76 ± 13.08 a | 2.61 ± 0.08 a,b | 2929.8 ± 8.8 d | 39.09 ± 0.96 a,b | 30.54 ± 0.75 a |
| 0.2 μm | 0.9455 ± 0.0001 c | 1194.87 ± 10.75 a | 2.56 ± 0.05 a | 3374.7 ± 79.5 a,b | 40.99 ± 1.01 b,c | 30.5 ± 0.75 a |
| SG | |||||
| Model Name | Variant | Constants | R2 | RMSE | |
| k | B | ||||
| Modified Penetration model | NF | 0.0354 | 0.2899 | 0.9968 | 0.0033 |
| 8 μm | 0.0541 | 0.2014 | 0.9961 | 0.0037 | |
| 5 μm | 0.0249 | 0.3802 | 0.9946 | 0.0046 | |
| 3 μm | 0.0211 | 0.4133 | 0.9934 | 0.0051 | |
| 1.2 μm | 0.0398 | 0.2721 | 0.9778 | 0.0089 | |
| 0.8 μm | 0.0273 | 0.3556 | 0.9899 | 0.0061 | |
| 0.45 μm | 0.0266 | 0.3505 | 0.9773 | 0.0088 | |
| 0.2 μm | 0.0373 | 0.2793 | 0.9717 | 0.0098 | |
| WL | |||||
| Model Name | Variant | Constants | R2 | RMSE | |
| k1 | k2 | ||||
| Peleg’s model | NF | 29.563 | 1.6359 | 0.9957 | 0.0149 |
| 8 μm | 23.808 | 1.7922 | 0.9974 | 0.0109 | |
| 5 μm | 24.051 | 1.8447 | 0.9974 | 0.0106 | |
| 3 μm | 26.845 | 1.7530 | 0.9832 | 0.0135 | |
| 1.2 μm | 27.262 | 1.7902 | 0.9854 | 0.0253 | |
| 0.8 μm | 27.231 | 1.7689 | 0.9997 | 0.0034 | |
| 0.45 μm | 20.354 | 1.8995 | 0.9990 | 0.0066 | |
| 0.2 μm | 24.260 | 1.7968 | 0.9685 | 0.0384 | |
| Time, min | NF | 8 μm | 5 μm | 3 μm | 1.2 μm | 0.8 μm | 0.45 μm | 0.2 μm | |
|---|---|---|---|---|---|---|---|---|---|
| TPC mg GA·100 g−1 dm | 0 | 151.66 ± 19.35 | 151.66 ± 19.35 | 151.66 ± 19.35 | 151.66 ± 19.35 | 151.66 ± 19.35 | 151.66 ± 19.35 | 151.66 ± 19.35 | 151.66 ± 19.35 |
| 15 | 495.88 ± 18.04 a,b | 493.69 ± 9.87 a | 560.16 ± 8.99 a–e | 604.69 ± 44.21 a–h | 510.39 ± 21.26 a–c | 566.85 ± 20.95 a–f | 496.72 ± 4.64 a,b | 544.82 ± 30.38 a–d | |
| 30 | 834.32 ± 33.26 f–k | 626.81 ± 6.75 a–h | 654.18 ± 5.93 a–h | 677.87 ± 39.65 a–h | 603.32 ± 57.9 a–h | 609.88 ± 8.74 a–h | 571.33 ± 25.8 a–g | 631.02 ± 61.97 a–h | |
| 60 | 861.6 ± 32.71 h–k | 799.33 ± 18.03 d–k | 771.03 ± 2.57 c–k | 691.88 ± 33.02 a–h | 697.99 ± 28.23 a–i | 767.33 ± 14.99 b–j | 803.05 ± 22.9 d–k | 789.38 ± 44.33 d–k | |
| 90 | 969.73 ± 46.24 i–k | 738.04 ± 57.29 a–i | 711.69 ± 33.76 a–i | 808.89 ± 15.48 d–k | 837.14 ± 24.77 f–k | 864.32 ± 35.48 h–k | 782.51 ± 27.09 c–k | 731.17 ± 5.37 a–i | |
| 120 | 1040.32 ± 31.95 k | 814.81 ± 35.49 d–k | 821.46 ± 13.08 e–k | 842.32 ± 358.73 g–k | 1017.11 ± 15.45 j,k | 788.99 ± 27.4 d–k | 805.23 ± 25.62 d–k | 822.19 ± 2.81 e–k | |
| TEAC ABTS mmol Trx·100 g−1 dm | 0 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 |
| 15 | 10.74 ± 0.26 w | 3.82 ± 0.09 a | 7.27 ± 0.18 l,m | 8.14 ± 0.2 n–p | 4.93 ± 0.12 c–e | 4.76 ± 0.12 b–d | 8.35 ± 0.21 o–r | 5.53 ± 0.14 e–h | |
| 30 | 12.69 ± 0.31 x | 6.45 ± 0.16 j | 5.98 ± 0.15 h–j | 10.75 ± 0.26 w | 8.6 ± 0.21 p–s | 4.32 ± 0.11 a,b | 7.77 ± 0.19 m–o | 5.62 ± 0.14 f–i | |
| 60 | 6.06 ± 0.15 h–j | 9.01 ± 0.22 s–u | 6.21 ± 0.15 i,j | 7.36 ± 0.18 l,m | 7.67 ± 0.19 l–n | 5.31 ± 0.13 c–g | 5.1 ± 0.13 c–f | 8.86 ± 0.22 r–t | |
| 90 | 9.35 ± 0.23 u–w | 5.67 ± 0.14 f–i | 10.63 ± 0.26 w | 6 ± 0.15 h–j | 8.39 ± 0.21 p,r | 6.5 ± 0.16 j–k | 7.4 ± 0.18 l,m | 4.73 ± 0.12 b–c | |
| 120 | 8.21 ± 0.2 n–p | 7.45 ± 0.18 l,m | 4.07 ± 0.1 a | 5.82 ± 0.14 g–i | 7.09 ± 0.17 k,l | 9.47 ± 0.23 u | 9.07 ± 0.22 s–u | 5.35 ± 0.13 d–g | |
| FRAP mmol Trx·100 g−1 dm | 0 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 |
| 15 | 9.36 ± 0.23 u | 3.64 ± 0.09 a,b | 5.79 ± 0.14 j–l | 6.92 ± 0.17 n–p | 4.65 ± 0.11 d–f | 4.57 ± 0.11 d–f | 6.99 ± 0.17 p–s | 4.83 ± 0.12 e–h | |
| 30 | 9.46 ± 0.23 u | 5.05 ± 0.12 f–h | 5.93 ± 0.15 k,l | 9.4 ± 0.23 u | 6.44 ± 0.16 m,n | 3.58 ± 0.09 a,b | 6.61 ± 0.16 o–r | 5.17 ± 0.13 g–i | |
| 60 | 5.57 ± 0.14 i–k | 7.1 ± 0.17 o–r | 4.81 ± 0.12 d–g | 6.46 ± 0.16 g–i | 6.26 ± 0.15 l,m | 4.01 ± 0.1 b,c | 4.88 ± 0.12 e–h | 7.38 ± 0.18 p–s | |
| 90 | 7.72 ± 0.19 o–r | 4.92 ± 0.12 e–h | 8.55 ± 0.21 t | 5.19 ± 0.13 g–i | 6.94 ± 0.17 n–p | 4.94 ± 0.12 e–h | 5.84 ± 0.14 k,l | 4.31 ± 0.11 c,d | |
| 120 | 7.05 ± 0.17 o–r | 6.14 ± 0.15 l,m | 3.5 ± 0.09 a | 5.32 ± 0.13 h–j | 6.26 ± 0.15 l,m | 7.36 ± 0.18 p–s | 7.47 ± 0.18 r,s | 4.53 ± 0.11 d,e |
| Time, min | NF | 8 μm | 5 μm | 3 μm | 1.2 μm | 0.8 μm | 0.45 μm | 0.2 μm | |
|---|---|---|---|---|---|---|---|---|---|
| TPC mg GA·100 g−1 dm | 0 | 3501.34 ± 102.93 k–p | 3257.38 ± 56.66 h–m | 3282.94 ± 73.44 i–m | 3293.4 ± 9.22 i–m | 3361.94 ± 87.5 i–o | 2681.18 ± 8.05 a–c | 2929.79 ± 8.77 c–g | 3374.71 ± 79.5 i–o |
| 15 | 3382.66 ± 82.37 i–o | 3356 ± 94.78 i–o | 3462.63 ± 51.77 j–o | 3132.31 ± 18 f–i | 3239.77 ± 116.4 h–l | 3520.35 ± 91.62 l–p | 2638.16 ± 43.22 a,b | 3515.33 ± 82.81 l–p | |
| 30 | 3273.42 ± 125.52 i–m | 3996.71 ± 69.46 s | 3440.35 ± 23.28 j–o | 3388.16 ± 29.11 i–o | 3476.6 ± 142.48 j–p | 3299.45 ± 118.55 i–m | 3634.83 ± 164.66 o–r | 3755.5 ± 66.41 p–s | |
| 60 | 2983.91 ± 27.39 d–h | 3252.74 ± 78.61 h–m | 3987.43 ± 28.28 s | 3142.78 ± 57.67 f–i | 2844.76 ± 8.54 a–e | 3893.54 ± 61.35 r,s | 3269.11 ± 26.8 h–m | 2559.59 ± 44.66 a | |
| 90 | 2739.99 ± 92.88 a–d | 3508.94 ± 27.26 k–p | 2892.58 ± 114.98 b–f | 3136.57 ± 124.74 f–i | 3586.06 ± 93.33 n–p | 3193.23 ± 9.56 g–j | 3343.37 ± 258.64 i–m | 3227.48 ± 50.96 h–k | |
| 120 | 2776.94 ± 85.51 a–d | 3983.12 ± 105.8 s | 3126.81 ± 53.74 e–i | 3541.29 ± 9.91 m–p | 3361.37 ± 120.77 i–o | 2659.61 ± 43.57 a–c | 3146.19 ± 37.14 f–i | 2600.88 ± 69.18 a | |
| TEAC ABTS mmol Trx·100 g−1 dm | 0 | 49.7 ± 1.22 w | 38.09 ± 0.94 g–m | 38.89 ± 0.96 i–p | 44.99 ± 1.11 t,u | 33.99 ± 0.84 c–e | 43.29 ± 1.06 s–t | 39.09 ± 0.96 i–r | 40.99 ± 1.01 l–s |
| 15 | 37.47 ± 0.92 f–j | 37.92 ± 0.93 f–l | 39.89 ± 0.98 j–r | 37.48 ± 0.92 f–k | 36.06 ± 0.89 c–i | 37.27 ± 0.92 f–j | 37.08 ± 0.91 e–j | 42.07 ± 1.03 p–t | |
| 30 | 38 ± 0.93 f–m | 48.08 ± 1.18 u,w | 41.17 ± 1.01 m–s | 41.38 ± 1.02 n–s | 41.55 ± 1.02 o–s | 32.93 ± 0.81 b,c | 35.96 ± 0.88 c–i | 30.58 ± 0.75 a,b | |
| 60 | 35.17 ± 0.86 c–g | 38.47 ± 0.95 h–o | 47.24 ± 1.16 u,w | 37.72 ± 0.54 f–k | 36.17 ± 0.89 d–i | 39.99 ± 0.98 j–r | 35.65 ± 0.88 c–h | 38.32 ± 0.94 g–n | |
| 90 | 32.98 ± 0.81 b–d | 43.56 ± 1.07 s,t | 37.17 ± 0.91 e–j | 38.48 ± 0.95 h–o | 37.53 ± 0.92 f–j | 37.7 ± 0.93 f–k | 34.82 ± 0.86 c–f | 29.66 ± 0.73 a | |
| 120 | 40.73 ± 1 k–s | 47.04 ± 1.16 u,w | 39.56 ± 0.97 j–r | 35.36 ± 0.87 c–h | 37.74 ± 0.93 f–k | 33.37 ± 0.82 b–d | 41.54 ± 1.02 o–s | 42.24 ± 1.04 r–t | |
| FRAP mmol Trx·100 g−1 dm | 0 | 32.9 ± 0.81 p–s | 32.9 ± 0.81 p–s | 32.51 ± 0.8 o–s | 30.73 ± 0.76 m–p | 31 ± 0.76 m–r | 35.26 ± 0.87 t,u | 30.54 ± 0.75 i–o | 30.5 ± 0.75 l–o |
| 15 | 28.04 ± 0.69 h–k | 28.76 ± 0.71 j–m | 30.36 ± 0.75 k–o | 26.34 ± 0.65 f–i | 29.92 ± 0.74 k–n | 29.63 ± 0.73 j–n | 28.05 ± 0.69 h–k | 30.68 ± 0.75 m–p | |
| 30 | 24.27 ± 0.6 b–f | 24.66 ± 0.61 b–f | 32.95 ± 0.81 p–t | 33.45 ± 0.82 s–t | 32.98 ± 0.81 p–t | 21.75 ± 0.53 a | 24.64 ± 0.61 b–f | 22.92 ± 0.56 a,b | |
| 60 | 22.77 ± 0.56 a,b | 23.58 ± 0.58 a–d | 36.03 ± 0.89 u | 27.44 ± 0.43 g–j | 25.95 ± 0.64 e–h | 30.96 ± 0.76 m–r | 28.32 ± 0.7 i–l | 29.29 ± 0.72 j–n | |
| 90 | 23.2 ± 0.57 a–c | 23.64 ± 0.58 a–e | 23.7 ± 0.58 a–e | 29.47 ± 0.72 j–n | 26.26 ± 0.65 f–i | 29.82 ± 0.73 k–n | 25.6 ± 0.63 d–g | 21.87 ± 0.54 a | |
| 120 | 23.18 ± 0.57 a–c | 23.62 ± 0.58 a–e | 29.29 ± 0.72 j–n | 26.3 ± 0.65 f–i | 33.08 ± 0.81 r–t | 25.3 ± 0.62 c–g | 29.12 ± 0.72 j–m | 31.62 ± 0.78 n–s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masztalerz, K.; Figiel, A.; Michalska-Ciechanowska, A.; Wojdyło, A.; Nowicka, P.; Lech, K. The Effect of Filtration on Physical and Chemical Properties of Osmo-Dehydrated Material. Molecules 2020, 25, 5412. https://doi.org/10.3390/molecules25225412
Masztalerz K, Figiel A, Michalska-Ciechanowska A, Wojdyło A, Nowicka P, Lech K. The Effect of Filtration on Physical and Chemical Properties of Osmo-Dehydrated Material. Molecules. 2020; 25(22):5412. https://doi.org/10.3390/molecules25225412
Chicago/Turabian StyleMasztalerz, Klaudia, Adam Figiel, Anna Michalska-Ciechanowska, Aneta Wojdyło, Paulina Nowicka, and Krzysztof Lech. 2020. "The Effect of Filtration on Physical and Chemical Properties of Osmo-Dehydrated Material" Molecules 25, no. 22: 5412. https://doi.org/10.3390/molecules25225412
APA StyleMasztalerz, K., Figiel, A., Michalska-Ciechanowska, A., Wojdyło, A., Nowicka, P., & Lech, K. (2020). The Effect of Filtration on Physical and Chemical Properties of Osmo-Dehydrated Material. Molecules, 25(22), 5412. https://doi.org/10.3390/molecules25225412