Carrageenan-Based Films Incorporated with Jaboticaba Peel Extract: An Innovative Material for Active Food Packaging
Abstract
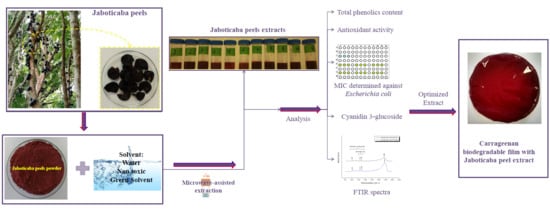
:1. Introduction
2. Results and Discussion
2.1. Extracts Optimization and Characterization
2.2. Biogegradable Films Characterization
3. Materials and Methods
3.1. Reagents
3.2. Sample Preparation
3.3. Extraction Procedures
3.4. Bioactive Compounds
3.5. Antimicrobial Inhibition (AI)
3.6. Fourier Transform Infrared Spectroscopy (FTIR-ATR)
3.7. Biodegradable Film Formation
3.8. Biodegradable Film Characterization
3.8.1. Film Thickness
3.8.2. Color Measurement
3.8.3. Solubility in Water
3.8.4. Mechanical Properties
3.9. Analysis of Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radaelli, J.C.; Porto, A.H.; Júnior, A.W.; Domingues, S.; Mazaro, S.M.; Citadin, I. Repeatability based on growth behavior of jabuticabeira tree genotypes. Rev. Bras. Frutic. 2018, 40, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Gurak, P.D.; De Bona, G.S.; Tessaro, I.C.; Marczak, L.D.F. Jaboticaba pomace powder obtained as a co-product of juice extraction: A comparative study of powder obtained from peel and whole fruit. Food Res. Int. 2014, 62, 786–792. [Google Scholar] [CrossRef]
- da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Tsaltaki, C.; Katsouli, M.; Kekes, T.; Chanioti, S.; Tzia, C. Comparison study for the recovery of bioactive compounds from Tribulus terrestris, Panax ginseng, Gingko biloba, Lepidium meyenii, Turnera diffusa and Withania somnifera by using microwave-assisted, ultrasound-assisted and conventional extraction methods. Ind. Crops Prod. 2019, 142, 111875. [Google Scholar] [CrossRef]
- Li, J.; Zu, Y.G.; Fu, Y.J.; Yang, Y.C.; Li, S.M.; Li, Z.N.; Wink, M. Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2010, 11, 637–643. [Google Scholar] [CrossRef]
- Abdou, E.S.; Sorour, M.A. Preparation and characterization of starch/carrageenan edible films. Int. Food Res. J. 2014, 21, 189–193. [Google Scholar]
- Robertson, G.L. Food Packaging: Principles and Practice, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined ĸ-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. Reinforcement of refined and semi-refined carrageenan film with nanocellulose. Polymers 2020, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Chi, W.; Cao, L.; Sun, G.; Meng, F.; Zhang, C.; Li, J.; Wang, L. Developing a highly pH-sensitive ĸ-carrageenan-based intelligent film incorporating grape skin powder via a cleaner process. J. Clean. Prod. 2020, 244, 118862. [Google Scholar] [CrossRef]
- Martiny, T.R.; Pacheco, B.S.; Pereira, C.M.P.; Mansilla, A.; Astorga-España, M.S.; Dotto, G.L.; Moraes, C.C.; Rosa, G.S. A novel biodegradable film based on κ-carrageenan activated with olive leaves extract. Food Sci. Nutr. 2020, 3147–3156. [Google Scholar] [CrossRef] [PubMed]
- Quatrin, A.; Pauletto, R.; Maurer, L.H.; Minuzzi, N.; Nichelle, S.M.; Carvalho, J.F.C.; Maróstica, M.R.; Rodrigues, E.; Bochi, V.C.; Emanuelli, T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019, 78, 59–74. [Google Scholar] [CrossRef]
- Faria, G.S.; Jardim, F.B.B.; da Silva, A.C.; Costa, L.L.; Abdalla, D.R. Caracterização química da casca de jabuticaba (Myrciaria jabuticaba) liofilizada e sua aplicação em leite fermentado potencialmente simbiótico. J. CiÊncias Bioméd. Saúde 2016, 2, 90–97. [Google Scholar]
- Calloni, C.; Agnol, R.D.; Martínez, L.S.; de Siqueira Marcon, F.; Moura, S.; Salvador, M. Jaboticaba (Plinia trunciflora (O. Berg) Kausel) fruit reduces oxidative stress in human fibroblasts cells (MRC-5). Food Res. Int. 2015, 70, 15–22. [Google Scholar] [CrossRef]
- Lima, A.D.J.B.; Corrêa, A.D.; Saczk, A.A.; Martins, M.P.; Castilho, R.O. Anthocyanins, pigment stability and antioxidant activity in jabuticaba [Myrciaria cauliflora (Mart.) O. Berg]. Rev. Bras. Frutic. 2011, 33, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F. Microwave-assisted extraction of saponin, phenolic and flavonoid compounds from Trigonella foenum-graecum seed based on two level factorial design. J. Appl. Res. Med. Aromat. Plants 2019, 14, 100212. [Google Scholar] [CrossRef]
- Chemat, F.; Cravotto, G. Microwave-assisted extraction for bioactive compound. Food Eng. Ser. 2013. [Google Scholar] [CrossRef]
- Machado, G.H.A.; Marques, T.R.; de Carvalho, T.C.L.; Duarte, A.C.; de Oliveira, F.C.; Gonçalves, M.C.; Piccoli, R.H.; Corrêa, A.D. Antibacterial activity and in vivo wound healing potential of phenolic extracts from jaboticaba skin. Chem. Biol. Drug Des. 2018, 92, 1333–1343. [Google Scholar] [CrossRef]
- Xiao, T.; Wu, Z.; Shi, Q.; Zhang, X.; Zhou, Y.; Yu, X.; Xiao, Y. A retrospective analysis of risk factors and outcomes in patients with extended-spectrum beta-lactamase-producing Escherichia coli bloodstream infections. J. Glob. Antimicrob. Resist. 2019, 17, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.C.; Doyle, M.P. Food as a vehicle for transmission of Shiga toxin-producing Escherichia coli. J. Food Prot. 2007, 70, 2426–2449. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.; Alencar, N.M.M.; Steel, C.J. Improvement of sensorial and technological characteristics of extruded breakfast cereals enriched with whole grain wheat flour and jabuticaba (Myrciaria cauliflora) peel. LWT-Food Sci. Technol. 2018, 90, 207–214. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Pereira, C.; Calhelha, R.C.; José Alves, M.; Abreu, R.M.V.; Barros, L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Jabuticaba residues (Myrciaria jaboticaba (Vell.) Berg) are rich sources of valuable compounds with bioactive properties. Food Chem. 2020, 309, 125735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza-Moreira, T.M.; Severi, J.A.; Santos, E.; Silva, V.Y.A.; Vilegas, W.; Salgado, H.R.N.; Pietro, R.C.L.R. Chemical and antidiarrheal studies of plinia cauliflora. J. Med. Food 2011, 14, 1590–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel-moral, S.; Borrás-linares, I.; Lozano-sánchez, J.; Arráez-Román, D.; Martínez-Ferez, A.; Segura-carretero, A. Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Caillet, S.; Côté, J.; Sylvain, J.F.; Lacroix, M. Antimicrobial effects of fractions from cranberry products on the growth of seven pathogenic bacteria. Food Control. 2012, 23, 419–428. [Google Scholar] [CrossRef]
- Sun, G.; Chi, W.; Xu, S.; Wang, L. Developing a simultaneously antioxidant and pH-responsive κ-carrageenan/hydroxypropyl methylcellulose film blended with Prunus maackii extract. Int. J. Biol. Macromol. 2020, 155, 1393–1400. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Development of Biodegradable Films with Improved Antioxidant Properties Based on the Addition of Carrageenan Containing Olive Leaf Extract for Food Packaging Applications. J. Polym. Environ. 2020, 28, 123–130. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Shankar, S.; Reddy, J.P.; Rhim, J.W.; Kim, H.Y. Preparation, characterization, and antimicrobial activity of chitin nanofibrils reinforced carrageenan nanocomposite films. Carbohydr. Polym. 2015, 117, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.T.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Souza, B.W.S.; Vicente, A.A. Synergistic effects between κ-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll. 2012, 29, 280–289. [Google Scholar] [CrossRef] [Green Version]
- Hanani, Z.A.N. Physicochemical characterization of kappa-carrageenan (Euchema cottoni) based films incorporated with various plant oils. Carbohydr. Polym. 2016. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of polyvinyl alcohol on the physicochemical properties of biodegradable starch films. Mater. Chem. Phys. 2020, 239. [Google Scholar] [CrossRef]
- Francisco, C.B.; Pellá, M.G.; Silva, O.A.; Raimundo, K.F.; Caetano, J.; Linde, G.A.; Colauto, N.B.; Dragunski, D.C. Shelf-life of guavas coated with biodegradable starch and cellulose-based films. Int. J. Biol. Macromol. 2020, 152, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ili Balqis, A.M.; Nor Khaizura, M.A.R.; Russly, A.R.; Nur Hanani, Z.A. Effects of plasticizers on the physicochemical properties of kappa-carrageenan films extracted from Eucheuma cottonii. Int. J. Biol. Macromol. 2017, 103, 721–732. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Munisamy, S.; Bhat, R. Effect of potassium hydroxide on rheological and thermo-mechanical properties of semi-refined carrageenan (SRC) films. Food Biosci. 2018, 26, 104–112. [Google Scholar] [CrossRef]
- Rovina, K.; Vonnie, J.M.; Shaeera, S.N.; Yi, S.X.; Halid, N.F.A. Development of biodegradable hybrid polymer film for detection of formaldehyde in seafood products. Sens. Bio-Sens. Res. 2020, 27, 100310. [Google Scholar] [CrossRef]
- Leite-Legatti, A.V.; Batista, A.G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Machado, A.R.T.; de Carvalho-Silva, L.B.; Ruiz, A.L.T.G.; et al. Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative Methods for Anthocyanins. 1. Extraction and Determination of Total Anthocyanin in Cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Edible Wheat Gluten Films: Influence of the Main Process Variables on Film Properties using Response Surface Methodology. J. Food Sci. 1992, 57, 190–195. [Google Scholar] [CrossRef]
- ASTM Standard D882-09 Standard test method for tensile properties of thin plastic sheeting. In Proceedings of the ASTM, International, West Conshohocken, PA, USA, 1 January 2009; Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D882-09.htm (accessed on 8 April 2020).
Sample Availability: Samples of the compounds are available from the authors. |




| x (°C) | y (min) | TP (mgGAE·g−1) (d.b.) | AA (%) | TA (mg·100g−1) (d.b.) | C-3-G (mgcyan·g−1) (d.b.) | |
|---|---|---|---|---|---|---|
| 1 | −1(60) | –1(2) | 283.52 ± 12.22 | 94.5 ± 0.05 | 238.66 ± 5.86 | 435.91 ± 1.16 |
| 2 | −1(60) | 1(6) | 319.82 ± 2.43 | 93.9 ± 0 | 141.79 ± 4.16 | 656.97 ± 0.93 |
| 3 | 1(100) | –1(2) | 347.84 ± 3.76 | 92.8 ± 0.9 | 164.11 ± 2.23 | 526.41 ± 0.44 |
| 4 | 1(100) | 1(6) | 346.08 ± 4.23 | 92.4 ± 0.8 | 126.41 ± 4.53 | 302.41 ± 0.35 |
| 5 | −1.41(52) | 0(4) | 230.06 ± 0.44 | 94.9 ± 0.02 | 62.66 ± 1.41 | 369.01 ± 0.16 |
| 6 | 1.41(108) | 0(4) | 356.93 ± 2.48 | 94.8 ± 0.1 | 80.55 ± 2.16 | 80.75 ± 0.03 |
| 7 | 0(80) | −1.41(1) | 348.92 ± 1.75 | 92.6 ± 0.6 | 208.33 ± 7.04 | 686.05 ± 0.22 |
| 8 | 0(80) | 1.41(7) | 357.26 ± 0.60 | 90.9 ± 0.6 | 203.25 ± 1.66 | 646.89 ± 0.60 |
| 9 | 0(80) | 0(4) | 347.58 ± 2.57 | 92.4 ± 0.2 | 154.54 ± 2.63 | 683.69 ± 12.74 |
| 10 | 0(80) | 0(4) | 357.65 ± 2.12 | 95.4 ± 0.2 | 157.69 ± 2.91 | 554.30 ± 5.48 |
| 11 | 0(80) | 0(4) | 360.12 ± 6.71 | 93.6 ± 0.4 | 173.49 ± 4.68 | 736.82 ± 5.56 |
| Sum of Squares | Degrees of Freedom | Fvalue | Ftabled | R2 | R2 Adjusted | |
|---|---|---|---|---|---|---|
| TP | ||||||
| Regression | 15,433.02 | 1 | 13.51 | 161.44 | 0.93 | 0.99 |
| Residual | 1141.63 | 1 | ||||
| Lack of fit | 1053.34 | 3 | 7.95 | 19.16 | ||
| Pure error | 88.29 | 2 | ||||
| Total | 16,574.65 | 10 | ||||
| TA | ||||||
| Regression | 22,078.16 | 3 | 3.95 | 9.27 | 0.79 | 0.99 |
| Residual | 5581.62 | 3 | ||||
| Lack of fit | 5375.34 | 3 | 17.37 | 19.16 | ||
| Pure error | 206.28 | 2 | ||||
| Total | 27,659.78 | 10 | ||||
| C-3-G | ||||||
| Regression | 451,997.1 | 1 | 55.11 | 161.44 | 0.98 | 0.99 |
| Residual | 8201.3 | 1 | ||||
| Lack of fit | 5336.1 | 3 | 1.24 | 19.16 | ||
| Pure error | 2865.2 | 2 | ||||
| Total | 460,198.4 | 10 |
| Exp Conditions | TP, Pred Response (mgGAE·g−1) (d.b.) | TP (mgGAE·g−1) (d.b.) | TA, Pred Response (mg·100g−1) (d.b.) | TA (mg·100g−1) (d.b.) | C-3-G, Pred Response (mgcyan·g−1) (d.b.) | C-3-G (mgcyan·g−1) (d.b.) |
|---|---|---|---|---|---|---|
| 80 °C, 1 min | 345.41 | 383.81 ± 7.41 | 245.38 | 207.96 ± 7.82 | 694.15 | 789.25 ± 3.02 |
| Film | Thickness (mm) | Solubility (%) | Elongation at Break (%) | Tensile Strength (MPa) |
|---|---|---|---|---|
| CAR–control | 0.032 a ± 0.0048 | 79.02 a ± 4.76 | 21.30 a ± 3.58 | 10.69 a ± 1.61 |
| CAR–JPE | 0.054 b ± 0.0055 | 82.84 a ± 10.57 | 28.26 a ± 3.69 | 6.08 a ± 0.33 |
| Optical Properties | ||||
| Film | L* | a* | b* | ΔE |
| CAR–control | 94.49 a ± 0.21 | −0.145 a ± 0.03 | 3.32 a ± 0.30 | - |
| CAR–JPE | 19.96 b ± 1.08 | 52.77 b ± 1.10 | 34.26 b ± 1.86 | 96.50 |
| Variables | Levels | ||||
| −1.41 | −1 | 0 | +1 | +1.41 | |
| Temperature (°C) | 51.8 | 60 | 80 | 100 | 108.2 |
| Time (min) | 1.18 | 2 | 4 | 6 | 6.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, L.B.; Barreto, E.R.C.; Souza, P.K.d.; Silva, B.D.Z.; Martiny, T.R.; Moraes, C.C.; Morais, M.M.; Raghavan, V.; Rosa, G.S.d. Carrageenan-Based Films Incorporated with Jaboticaba Peel Extract: An Innovative Material for Active Food Packaging. Molecules 2020, 25, 5563. https://doi.org/10.3390/molecules25235563
Avila LB, Barreto ERC, Souza PKd, Silva BDZ, Martiny TR, Moraes CC, Morais MM, Raghavan V, Rosa GSd. Carrageenan-Based Films Incorporated with Jaboticaba Peel Extract: An Innovative Material for Active Food Packaging. Molecules. 2020; 25(23):5563. https://doi.org/10.3390/molecules25235563
Chicago/Turabian StyleAvila, Luisa Bataglin, Elis Regina Correa Barreto, Paloma Krolow de Souza, Bárbara De Zorzi Silva, Thamiris Renata Martiny, Caroline Costa Moraes, Marcilio Machado Morais, Vijaya Raghavan, and Gabriela Silveira da Rosa. 2020. "Carrageenan-Based Films Incorporated with Jaboticaba Peel Extract: An Innovative Material for Active Food Packaging" Molecules 25, no. 23: 5563. https://doi.org/10.3390/molecules25235563
APA StyleAvila, L. B., Barreto, E. R. C., Souza, P. K. d., Silva, B. D. Z., Martiny, T. R., Moraes, C. C., Morais, M. M., Raghavan, V., & Rosa, G. S. d. (2020). Carrageenan-Based Films Incorporated with Jaboticaba Peel Extract: An Innovative Material for Active Food Packaging. Molecules, 25(23), 5563. https://doi.org/10.3390/molecules25235563