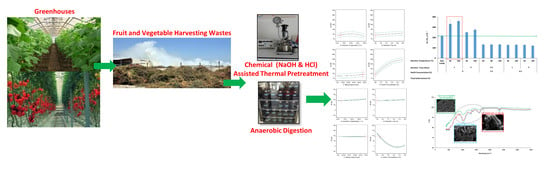
Impacts of Chemical-Assisted Thermal Pretreatments on Methane Production from Fruit and Vegetable Harvesting Wastes: Process Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of FVHW and Characterization Analysis
2.2. Chemical-Assisted Thermal Pretreatments Experiments
2.3. Biochemical Methane Potential (BMP)
2.4. Surface Properties and Bond Structure
3. Results and Discussion
3.1. Impacts of Chemical Assisted Thermal Pretreatments
3.2. Optimization of Chemical Assisted Thermal Pretreatment Process for sCOD and sSugar
3.3. Methane Production
3.4. Surface Modification and Molecular Bond Changes in FVHW by Chemical Assisted Thermal Pretreatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shanthi, M.; Rajesh, B.J.; Sivashanmugam, P. Synergistic effect of combined pretreatment in solubilizing fruits and vegetable residue for biogas production: Hydrolysis, energy assessment. Fuel 2019a, 250, 194–202. [Google Scholar] [CrossRef]
- Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 2 November 2019).
- Us, E.; Perendeci, A. Improvement of methane production from greenhouse residues: Optimization of thermal and H2SO4 pretreatment process by experimental design. Chem. Eng. J. 2012, 181, 120–131. [Google Scholar] [CrossRef]
- Calabro, P.S.; Gori, M.; Lubello, C. European trends in greenhouses gases emissions from integrated solid waste management. Environ. Technol. 2015, 36, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.; Elsamadony, M.; Mostafa, A.; Awad, H.; Tawfik, A. Harvesting zero waste from co-digested fruit and vegetable peels via integrated fermentation and pyrolysis processes. Environ. Sci. Pollut. Res. 2019, 26, 10429–10438. [Google Scholar] [CrossRef]
- Ceylan, R.F.; Sayin, C.; Yelboğa, M.N.M.; Özalp, M.; İlbasmiş, E.; Sav, O. Land ownership and profitability of greenhouse production: Antalya Case. Turk. J. Agric. Food Sci. Technol. 2009, 6, 930–935. [Google Scholar] [CrossRef]
- Dahunsi, S.O. Liquefaction of pineapple peel: Pretreatment and process optimization. Energy 2019, 185, 1017–1031. [Google Scholar] [CrossRef] [Green Version]
- Chandra, R.; Takeuchi, H.; Hasegaw, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T.; Kumar, R. Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energy 2012, 43, 273–282. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Nguyen, D.; Surendra, K.C.; Shrestha, S.; Rajendran, K.; Oechsner, H.; Xie, L.; Khanal, S.K. Anaerobic biorefinery: Current status, challenges, and opportunities. Bioresour. Technol. 2016, 215, 304–313. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production from Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanthi, M.; Banu, J.R.; Sivashanmugam, P. Solubilization of fruits and vegetable dregs through surfactant mediated sonic disintegration: Impact on biomethane potential and energy ratio. Environ. Technol. 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Perendeci, N.A.; Gökgöl, S.; Orhon, D. Impact of alkaline H2O2 pretreatment on methane generation potential of greenhouse crop waste under anaerobic conditions. Molecules 2018, 23, 1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeynali, R.; Khojastehpour, M.; Ebrahimi, -N.M. Effect of ultrasonic pre-treatment on biogas yield and specific energy in anaerobic digestion of fruit and vegetable wholesale market wastes. Sustain. Environ. Res. 2017, 27, 259–264. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Li, J.; Li, H.; Yu, Z. Effects of thermal pretreatment on the biomethane yield and hydrolysis rate of kitchen waste. Appl. Energy 2016, 172, 47–58. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2005.
- Van Soest, P.J. Use of detergent in the analysis of fibrous feeds. A rapid method for the determination of fibre and lignin. J. Assoc. Off. Anal. Chem. 1963, 46, 829–835. [Google Scholar]
- Dreywood, R. Qualitative test for carbohydrate material. Ind. Eng. Chem. Res. 1946, 18, 199. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Fau, A.L.; Randall, R.J. Protein measurement with the Folin reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Bridoux, G.; Dhulster, P.; Manem, J. Grease analysis on municipal wastewater treatment. Plants Tech. Sci. Methodes 1994, 5, 257–262. [Google Scholar]
- Wang, Z.; Keshwani, D.R.; Redding, A.P.; Cheng, J.J. Sodium hydroxide pretreatment and enzymatic hydrolysis of coastal Bermuda grass. Bioresour. Technol. 2010, 101, 3583–3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, S.J.M.; Villa, R.M.S.; Veloz, R.J.S.; Rivera, H.K.N.; González, C.R.A.; Plascencia, E.M.A.; Trejo, E.S.R. Comparative hydrolysis and fermentation of sugarcane and agave bagasse. Bioresour. Technol. 2009, 100, 1238–1245. [Google Scholar]
- Sukumaran, R.K.; Singhania, R.R.; Mathew, G.M.; Pandey, A. Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew. Energy 2009, 34, 421–424. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, F.; Cheng, K.; Liua, D. Enhancement of the enzymatic digestibility of sugarcane bagasse by alkali–peracetic acid pretreatment. Enzym. Microb. Technol. 2009, 44, 17–23. [Google Scholar] [CrossRef]
- Darani, K.K.; Zoghi, A. Comparison of pretreatment strategies of sugarcane baggase: Experimental design for citric acid production. Bioresour. Technol. 2008, 99, 9686–9993. [Google Scholar]
- Mottet, A.; Steyer, J.P.; Deleris, S.; Vedrenne, F.; Chauzy, J.; Carrère, H. Kinetics of thermophilic batch anaerobic digestion of thermal hydrolysed waste activated sludge. Biochem. Eng. J. 2009, 46, 169–175. [Google Scholar] [CrossRef]
- Ozay, Y.; Kokdemir, U.E.; İsik, Z.; Yılmaz, F.; Dizge, N.; Perendeci, N.A.; Mazmanci, M.A.; Yalvac, M. Optimization of electrocoagulation process and combination of anaerobic digestion for the treatment of pistachio processing wastewater. J. Clean. Prod. 2018, 196, 42–50. [Google Scholar] [CrossRef]
- Carrere, H.; Sialve, B.; Bernet, N. Improving pig manure conversion into biogas by thermal and thermo-chemical pretreatments. Bioresour. Technol. 2009, 100, 3690–3694. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenes, J.P.; Carrere, H. Effects of thermal treatments on five different waste activated sludge samples solubilization, physical properties and anaerobic digestion. Chem. Eng. J. 2008, 139, 236–244. [Google Scholar] [CrossRef]
- Teghammar, A.; Yngvesson, J.; Lundin, M.; Taherzadeh, M.J.; Horvath, I.S. Pretreatment of paper tube residues for improved biogas production. Bioresour. Technol. 2010, 101, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Lu, W.; Zhao, Y. Digestibility improvement of sorted waste with alkaline hydrothermal pretreatment. Tsinghua Sci. Technol. 2009, 14, 378–382. [Google Scholar] [CrossRef]
- Jard, G.; Dumas, C.; Delgenes, J.P.; Marfaing, H.; Sialvec, B.; Steyer, J.P.; Carrèrea, H. Effect of thermochemical pretreatment on the solubilization and anaerobic biodegradability of the red macroalga Palmaria palmata. Biochem. Eng. J. 2013, 79, 253–258. [Google Scholar] [CrossRef]
- Yılmaz, F.; Kökdemir, U.E.; Perendeci, N.A. Enhancement of methane production from banana harvesting residues: Optimization of thermal-alkaline hydrogen peroxide pretreatment process by experimental design. Waste Biomass Valorization 2019, 10, 3071–3087. [Google Scholar]
- Chen, H.; Zhang, C.; Rao, Y.; Jing, Y.; Luo, G.; Zhang, S. Methane potentials of wastewater generated from hydrothermal liquefaction of rice straw: Focusing on the wastewater characteristics and microbial community compositions. Biotechnol. Biofuels 2017, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Larroche, C.; Dussap, C.G.; Gnansounou, E.; Khanal, S.K.; Ricke, S. Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Song, X.; Shi, Z.; Li, X.; Wang, X.; Ren, Y. Fate of proteins of waste activated sludge during thermal alkali pretreatment in terms of sludge protein recovery. Front. Environ. Sci. Eng. 2019, 13, 25. [Google Scholar] [CrossRef]
- Speece, R.E. Anaerobic Biotechnology for Industrial Wastewaters; Archae Press: Nashville, TN, USA, 1996. [Google Scholar]
- Song, Z.; Yang, G.; Liu, X.; Yan, Z.; Yuan, Y.; Liao, Y. Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE 2014, 9, e93801. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Steyer, J.P.; Carrere, H. Comparison of seven types of thermos-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour. Technol. 2012, 120, 241–247. [Google Scholar] [CrossRef]
- Saikia, B.J.; Parthasarathy, G. Fourier Transform Infrared Spectroscopic Characterization of Kaolinite from Assam and Meghalaya, Northeastern India. J. Mod. Phys. 2010, 01, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Sumayya, A.; Panicker, C.Y.; Varghese, H.T.; Harikumar, B. Vibrational spectroscopic studies and AB inition calculations of l-glutamic acid 5-amide. Rjc Rasayanj. Chem. 2008, 1, 548–555. [Google Scholar]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Ang, N.; Ngoh, G.C.; Seak, A.; Chua, M.; Lee, M.G. Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses. Biotechnol. Biofuels 2012, 5, 1–10. [Google Scholar]
- Xu, X.J.-K.; Sun, Y.-C.; Sun, R.-C. Structural and hydrolysis characteristics of cypress pretreated by ionic liquids in a microwave irradiation environment. Bioenergy Res. 2014, 7, 1305–1316. [Google Scholar] [CrossRef]
- Fu, S.F.; Wang, F.; Yuan, X.Z.; Yanf, Z.M.; Luo, S.J.; Wang, C.S.; Guo, R.B. The thermophilic (55 °C) microaerobic pretreatment of corn straw for anaerobic digestion. Bioresour. Technol. 2015, 175, 203–208. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Cao, W.; Yin, R.; Mei, Y.; Zhang, L. Impacts of alkaline hydrogen peroxide pretreatment on chemical composition and biochemical methane potential of agricultural crop stalks. Energy Fuels 2015, 29, 4966–4975. [Google Scholar] [CrossRef]
- Gerakines, P.; Schutte, W.A.; Greenberg, J.M.; van Dishoeck, E.F. The infrared band strengths of H2O, CO and CO2 in laboratory simulations of astrophysical ice mixtures. Astr. Astrophys. 1995, 2996, 810–818. [Google Scholar]
Sample availability: Samples of the compounds are not available from the authors. |





| NaOH-Assisted Thermal Pretreatment | |||
|---|---|---|---|
| Independent Variables | Coded Variable Levels | ||
| Minimum (−1) | Center (0) | Maximum (+1) | |
| A: Reaction Temperature, (°C) | 60 | 80 | 100 |
| B: Reaction Time, (Hour) | 1 | 2.5 | 4 |
| C: Mixing Speed, (rpm) | 0 | 250 | 500 |
| D: NaOH Concentration, (%, w/v) | 0 | 3.25 | 6.5 |
| E: Dry Matter (DM) Content, (%) | 5 | ||
| HCl-Assisted Thermal Pretreatment | |||
| Independent Variables | Coded Variable Levels | ||
| Minimum (−1) | Center (0) | Maximum (+1) | |
| A: HCl Concentration, (%, w/v) | 0 | 2.5 | 5 |
| B: Reaction Temperature, (°C) | 60 | 80 | 100 |
| C: Dry Matter (DM) Content (%) | 1 | 2.5 | 5 |
| D: Mixing Speed, (rpm) | 0 | 250 | 500 |
| E: Reaction Time, (Hour) | 1 | ||
| Parameters | Value |
|---|---|
| Total Solid, TS, g TS/kg dry sample | 913.93 |
| Volatile Solid, VS, g VS/kg TS | 694.09 |
| Total Kjeldahl Nitrogen, TKN, g N/kg VS | 24.80 |
| Total Organic Carbon, g TOC/kg VS | 501.77 |
| Soluble Protein (g SProtein/kg VS) | 94.56 |
| Soluble Sugar (g SGlucose/kg VS) | 279.50 |
| Extractable Material and Lipid (g/kgVS) | 42.17 |
| Van Soest Fractionation (TS basis) | |
| Celulose, % | 33.12 |
| Hemicellulose, % | 23.74 |
| Lignin, % | 9.32 |
| Soluble matter, % | 33.82 |
| Elemental Analysis (TS basis) | |
| Carbon, C, % | 34.16 |
| Hydrogen, H, % | 5.03 |
| Nitrogen, N, % | 2.39 |
| Sulphur, S, % | 0.82 |
| C/N | 14.29 |
| NaOH-Assisted Thermal Pretreatment | |||||
| Source | Sum of Square | Degrees of Freedom | Mean Square | F-Value | Prob > F |
| sCOD | |||||
| Model | 4.907 × 106 | 14 | 3.505 × 105 | 53.31 | <0.0001 |
| A—Reaction Temperature | 43898.63 | 1 | 43898.63 | 6.68 | 0.0136 |
| B—Reaction Time | 23782.27 | 1 | 23782.27 | 3.62 | 0.0646 |
| C—Mixing Speed | 29589.73 | 1 | 29589.73 | 4.50 | 0.0403 |
| D—NaOH Concentration | 4.147 × 106 | 1 | 4.147 × 106 | 630.71 | <0.0001 |
| AB | 5767.11 | 1 | 5767.11 | 0.8772 | 0.3547 |
| AC | 10885.13 | 1 | 10885.13 | 1.66 | 0.2058 |
| AD | 62377.77 | 1 | 62377.77 | 9.49 | 0.0038 |
| BC | 553.20 | 1 | 553.20 | 0.0841 | 0.7733 |
| BD | 3.86 | 1 | 3.86 | 0.0006 | 0.9808 |
| CD | 245.92 | 1 | 245.92 | 0.0374 | 0.8476 |
| A² | 54067.88 | 1 | 54067.88 | 8.22 | 0.0066 |
| B² | 0.3052 | 1 | 0.3052 | 0.0000 | 0.9946 |
| C² | 1573.40 | 1 | 1573.40 | 0.2393 | 0.6274 |
| D² | 1.185 × 105 | 1 | 1.185 × 105 | 18.02 | 0.0001 |
| R2 = 0.9503, Adj-R2 = 0.9325, Adeq Precision = 20.0622 C.V. % = 8.33 | |||||
| HCl-Assisted Thermal Pretreatment | |||||
| Source | Sum of Square | Degrees of Freedom | Mean Square | F-Value | Prob > F |
| sCOD | |||||
| Model | 2.104 × 106 | 14 | 1.503 × 105 | 36.35 | <0.0001 |
| A—Reaction Temperature | 4.591 × 105 | 1 | 4.591 × 105 | 111.09 | <0.0001 |
| B—HCl Concentration | 1.132 × 106 | 1 | 1.132 × 106 | 273.80 | <0.0001 |
| C—Solid Content | 69230.38 | 1 | 69230.38 | 16.75 | 0.0002 |
| D—Mixing Speed | 10722.26 | 1 | 10722.26 | 2.59 | 0.1153 |
| AB | 2.004 × 105 | 1 | 2.004 × 105 | 48.49 | <0.0001 |
| AC | 13407.44 | 1 | 13407.44 | 3.24 | 0.0794 |
| AD | 604.22 | 1 | 604.22 | 0.1462 | 0.7043 |
| BC | 1.182 × 105 | 1 | 1.182 × 105 | 28.60 | <0.0001 |
| BD | 216.27 | 1 | 216.27 | 0.0523 | 0.8203 |
| CD | 5219.10 | 1 | 5219.10 | 1.26 | 0.2680 |
| A² | 18583.84 | 1 | 18583.84 | 4.50 | 0.0404 |
| B² | 11063.97 | 1 | 11063.97 | 2.68 | 0.1099 |
| C² | 4855.75 | 1 | 4855.75 | 1.17 | 0.2851 |
| D² | 20711.11 | 1 | 20711.11 | 5.01 | 0.0310 |
| R2 = 0.9288, Adj-R2 = 0.9033, Adeq Precision = 22.3369, C.V. % = 8.53 | |||||
| NaOH-Assisted Thermal Pretreatment | |||||
| Source | Sum of Square | Degrees of Freedom | Mean Square | F-Value | Prob > F |
| sSugar | |||||
| Model | 8.671 × 105 | 14 | 61936.12 | 854.31 | <0.0001 |
| A—Reaction Temperature | 2058.59 | 1 | 2058.59 | 28.40 | <0.0001 |
| B—Reaction Time | 360.68 | 1 | 360.68 | 4.98 | 0.0315 |
| C—Mixing Speed | 83.78 | 1 | 83.78 | 1.16 | 0.2890 |
| D—NaOH Concentration | 6.434 × 105 | 1 | 6.434 × 105 | 8874.32 | <0.0001 |
| AB | 42.60 | 1 | 42.60 | 0.5876 | 0.4480 |
| AC | 117.12 | 1 | 117.12 | 1.62 | 0.2112 |
| AD | 3549.03 | 1 | 3549.03 | 48.95 | <0.0001 |
| BC | 8.04 | 1 | 8.04 | 0.1109 | 0.7409 |
| BD | 1264.29 | 1 | 1264.29 | 17.44 | 0.0002 |
| CD | 50.10 | 1 | 50.10 | 0.6911 | 0.4109 |
| A² | 207.77 | 1 | 207.77 | 2.87 | 0.0985 |
| B² | 67.99 | 1 | 67.99 | 0.9378 | 0.3388 |
| C² | 12.02 | 1 | 12.02 | 0.1657 | 0.6862 |
| D² | 82123.05 | 1 | 82123.05 | 1132.76 | <0.0001 |
| R2 = 0.9967, Adj-R2 = 0.9956, Adeq Precision = 69.1844, C.V. % = 7.24 | |||||
| HCl-Assisted Thermal Pretreatment | |||||
| Source | Sum of Square | Degrees of Freedom | Mean Square | F-Value | Prob > F |
| sSugar | |||||
| Model | 2.318 × 106 | 14 | 1.656 × 105 | 30.43 | <0.0001 |
| A—Reaction Temperature | 30752.88 | 1 | 30752.88 | 5.65 | 0.0224 |
| B—HCl Concentration | 2.588 × 105 | 1 | 2.588 × 105 | 47.56 | <0.0001 |
| C—Solid Content | 43307.69 | 1 | 43307.69 | 7.96 | 0.0075 |
| D—Mixing Speed | 8252.21 | 1 | 8252.21 | 1.52 | 0.2255 |
| AB | 2294.35 | 1 | 2294.35 | 0.4217 | 0.5199 |
| AC | 45.89 | 1 | 45.89 | 0.0084 | 0.9273 |
| AD | 512.64 | 1 | 512.64 | 0.0942 | 0.7605 |
| BC | 1.395 × 105 | 1 | 1.395 × 105 | 25.64 | <0.0001 |
| BD | 1547.35 | 1 | 1547.35 | 0.2844 | 0.5969 |
| CD | 1193.16 | 1 | 1193.16 | 0.2193 | 0.6422 |
| A² | 19378.84 | 1 | 19378.84 | 3.56 | 0.0666 |
| B² | 6.606 × 105 | 1 | 6.606 × 105 | 121.41 | <0.0001 |
| C² | 21405.59 | 1 | 21405.59 | 3.93 | 0.0544 |
| D² | 7628.98 | 1 | 7628.98 | 1.40 | 0.2435 |
| R2 = 0.9161, Adj-R2 = 0.8860, Adeq Precision = 16.5818, C.V. % = 26.19 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günerhan, Ü.; Us, E.; Dumlu, L.; Yılmaz, V.; Carrère, H.; Perendeci, A.N. Impacts of Chemical-Assisted Thermal Pretreatments on Methane Production from Fruit and Vegetable Harvesting Wastes: Process Optimization. Molecules 2020, 25, 500. https://doi.org/10.3390/molecules25030500
Günerhan Ü, Us E, Dumlu L, Yılmaz V, Carrère H, Perendeci AN. Impacts of Chemical-Assisted Thermal Pretreatments on Methane Production from Fruit and Vegetable Harvesting Wastes: Process Optimization. Molecules. 2020; 25(3):500. https://doi.org/10.3390/molecules25030500
Chicago/Turabian StyleGünerhan, Ümmihan, Ender Us, Lütfiye Dumlu, Vedat Yılmaz, Hélène Carrère, and Altınay N. Perendeci. 2020. "Impacts of Chemical-Assisted Thermal Pretreatments on Methane Production from Fruit and Vegetable Harvesting Wastes: Process Optimization" Molecules 25, no. 3: 500. https://doi.org/10.3390/molecules25030500
APA StyleGünerhan, Ü., Us, E., Dumlu, L., Yılmaz, V., Carrère, H., & Perendeci, A. N. (2020). Impacts of Chemical-Assisted Thermal Pretreatments on Methane Production from Fruit and Vegetable Harvesting Wastes: Process Optimization. Molecules, 25(3), 500. https://doi.org/10.3390/molecules25030500