Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications
Abstract
:1. Introduction
2. Brown Seaweed Polysaccharides
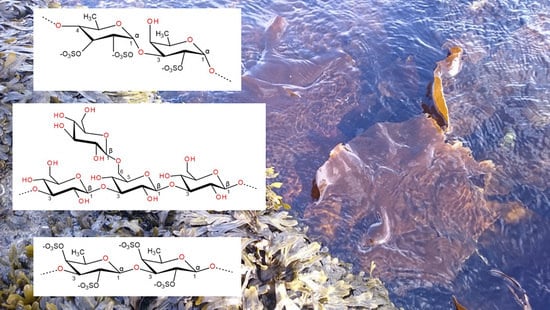
2.1. Fucoidan
2.2. Alginate
2.3. Laminarin
3. Red Seaweed Polysaccharides
3.1. Carrageenan
3.2. Agar
4. Green Seaweed Polysaccharides
5. Extraction of Seaweed Polysaccharides
5.1. Extraction of Brown Seaweed Polysaccharides
5.2. Extraction of Red Seaweed Polysaccharides
5.3. Extraction of Green Seaweed Polysaccharides
6. Co-Extraction of Seaweed Byproducts
7. Modification of Seaweed Polysaccharides
7.1. Fucoidan
7.2. Alginate
7.3. Laminarin
7.4. Carrageenan
7.5. Agar
7.6. Ulvan
8. Current and Potential Novel Applications
8.1. Fucoidan
8.2. Alginate
8.3. Laminarin
8.4. Carrageenan
8.5. Agar
8.6. Ulvan
9. Conclusions and Future Aspects
Author Contributions
Funding
Conflicts of Interest
References
- Chapman, V.J.; Chapman, D.J. Seaweeds and their Uses, 3rd ed.; Chapman and Hall Ltd.: London, UK, 1980. [Google Scholar] [CrossRef]
- FAO. FAO yearbook. Fishery and Aquaculture Statistics 2017; FAO: Rome, Italy, 2019. [Google Scholar]
- Campbell, I.; Macleod, A.; Sahlmann, C.; Neves, L.; Funderud, J.; Øverland, M.; Hughes, A.D.; Stanley, M. The Environmental Risks Associated With the Development of Seaweed Farming in Europe—Prioritizing Key Knowledge Gaps. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- FAO. The Global Status of Seaweed Production, Trade and Utilization; Globefish Research Programme: Rome, Italy, 2018; Volume 124, p. 120. [Google Scholar]
- Jung, K.A.; Lim, S.-R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.-G.; Toth, G.; Undeland, I. Production of protein extracts from Swedish red, green and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linneus, and Saccharina latissima (Linneus), J.V. Lamoroux, using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef] [Green Version]
- Hreggviðsson, G.O.; Nordberg-Karlsson, E.M.; Tøndervik, A.; Aachmanne, F.L.; Dobruchowska, J.M.; Linares-Pastén, J.; Daugbjerg-Christensen, M.; Moneart, A.; Kristjansdottir, T.; Slettad, H.; et al. Biocatalytic refining of polysaccharides from brown seaweeds. In Sustainable Seaweed Technologies—Cultivation, Biorefinery, and Applications, 1st ed.; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Milledge, J.J.; Harvey, P.J. Anaerobic Digestion and Gasification of Seaweed. In Grand Challenges in Marine Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 237–258. [Google Scholar] [CrossRef]
- Vijayaraghavan, M.R.; Sokhi, G. Phaeophyceae—An Ultrastructural and Histochemical Overview. Proc. Indian. Natn. Sci. Acad. 1986, 4, 529–546. [Google Scholar]
- Mautner, H.G. The Chemistry of Brown Algae. Econ. Bot. 1954, 8, 174–192. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Hervé, C. Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 2014, 114, 1203–1216. [Google Scholar] [CrossRef] [Green Version]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohyd. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Graiff, A.; Ruth, W.; Kragl, U.; Karsten, U. Chemical characterization and quantification of the brown algal storage compound laminarin - A new methodological approach. J. Appl. Phycol. 2016, 28, 533–543. [Google Scholar] [CrossRef]
- Kloareg, B.; Quatrano, R.S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. Annu. Rev. 1988, 26, 259–315. [Google Scholar]
- Salmeán, A.A.; Duffieux, D.; Harholt, J.; Qin, F.; Michel, G.; Czjzek, M.; Hervé, C. Insoluble (1→3), (1→4)-β-D-glucan is a component of cell walls in brown algae (Phaeophyceae) and is masked by alginates in tissues. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Mustapha, W.A.W.; Maskat, M.Y.; Latip, J.; Badri, K.H.; Hassan, O. Chemical Properties and Toxicology Studies of Fucoidan Extracted from Malaysian Sargassum binderi. Food Sci. Biotechnol. 2016, 25, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Starko, S.; Mansfield, S.D.; Martone, T. Cell wall chemistry and tissue structure underlie shifts in material properties of a perennial kelp. Eur. J. Phycol. 2018, 53, 307–317. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Kim, W.J.; Park, Y.I. Cell Wall Polysaccharides of Marine Algae. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin, Germany, 2015; pp. 543–590. [Google Scholar]
- Zhao, X.; Jiao, G.; Wu, J.; Zhang, J.; Yu, G. Laminaria japonica Aresch. and Ecklonia Kurome Okam. 昆布 (Kunbu, Kelp). In Dietary Chinese Herbs; Liu, Y., Wang, Z., Zhang, J., Eds.; Springer: Vienna, Austria, 2015. [Google Scholar]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohyd. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Tuvikene, R.; Truus, K.; Robal, M.; Volobujeva, O.; Mellikov, E.; Pehk, T.; Kollist, A.; Kailas, T.; Vaher, M. The extraction, structure, and gelling properties of hybrid galactan from the red alga Furcellaria lumbricalis (Baltic Sea, Estonia). J. Appl. Phycol. 2010, 22, 51–63. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Kidgell, J.T.; Magnusson, M.; deNys, R.; Glasson, R.K. Ulvan: A systematic review on extraction, composition and function. Algal Res. 2019, 39. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Xue, C.; Sun, L. Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. J. Appl. Phycol. 2012, 24, 295–300. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Seal, C.J.; Wilcox, M.; Dettmar, P.W.; Pearson, J.P. Applications of Alginates in Food. In Alginates: Biology and Applications; Rehm, B.H.A., Ed.; Springer-Verlag Berlin: Heidelberg, Germany, 2009; pp. 211–228. [Google Scholar]
- Miller, I.J. Alginate Composition of Some New Zealand Brown Seaweeds. Phytochemistry 1996, 41, 1315–1317. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Donati, I.; Paoletti, S. Alginate hydrogels: Properties and applications. In Polysaccharide Hydrogels: Characterization and Biomedical Applications; Matricardi, P., Alhaique, F., Coviello, T., Eds.; Pan Stanford Publishing Pte Ltd.: Singapore, 2015. [Google Scholar]
- Domozych, D. Algal Cell Wall. In Encyclopedia of Life Sciences, 4th ed.; John Wieley & Sons Ltd.: Chichester, UK, 2019; pp. 1–11. [Google Scholar]
- Devillé, C.; Damas, J.; Forget, P.; Dandrifosse, G.; Peulen, O. Laminarin in the dietary fibre concept. J. Sci. Food Agric. 2004, 84, 1030–1038. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Tech. 2014, 50, 24–31. [Google Scholar] [CrossRef]
- Read, S.M.; Currie, G.; Bacic, A. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr. Res. 1996, 23, 187–201. [Google Scholar] [CrossRef]
- Maeda, M.; Nisizawa, K. Fine structure of laminaran of Eisenia bicyclis. J. Biochem. 1968, 63, 199–206. [Google Scholar] [CrossRef]
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W.; et al. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Nelson, T.E.; Lewis, B.A. Separation and characterization of the soluble and insoluble components of insoluble laminaran. Carbohydr. Res. 1974, 33, 63–74. [Google Scholar] [CrossRef]
- Viola, R.; Nyvall, P.; Pedersén, M. The unique features of starch metabolism in red algae. Proc. Biol. Sci. 2001, 268, 1417–1422. [Google Scholar] [CrossRef] [Green Version]
- Amimi, A.; Mouradi, A.; Givernaud, T.; Chiadmi, N.; Lahaye, M. Structural analysis of Gigartina pistillata carrageenans (Gigartinaceae, Rhodophyta). Carbohyd. Res. 2001, 333, 271–279. [Google Scholar] [CrossRef]
- Running, C.A.; Falshaw, R.; Janaswamy, S. Trivalent iron induced gelation in lambda-carrageenan. Carbohyd. Polym. 2012, 87, 2735–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuhara, G.J.; Praseptiangga, D.; Riyanto, R.A. Extraction and Characterization of Refined K-carrageenan of Red Algae [Kappaphycus Alvarezii (Doty ex P.C. Silva, 1996)] Originated from Karimun Jawa Islands. Aquat. Procedia 2016, 7, 106–111. [Google Scholar] [CrossRef]
- Lebbar, S.; Fanuel, M.; Le Gall, S.; Falourd, X.; Ropartz, D.; Bressollier, P.; Gloaguen, V.; Faugeron-Girard, C. Agar Extraction By-Products from Gelidium sesquipedale as a Source of Glycerol-Galactosides. Molecules 2018, 23, 3364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.-L. Biosynthesis of agar in red seaweeds: A review. Carbohyd. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.D.; Verbruggen, H.; Delwiche, C.F.; De Clerck, O. Phylogeny and Molecular Evolution of the Green Algae. Crit. Rev. Plant. Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Sardari, R.R.R.; Nordberg Karlsson, E. Marine Poly- and Oligosaccharides as Prebiotics. J. Agric. Food Chem. 2018, 66, 11544–11549. [Google Scholar] [CrossRef]
- Wichard, T.; Charrier, B.; Mineur, F.; Bothwell, J.H.; Clerck, O.D.; Coates, J.C. The green seaweed Ulva: A model system to study morphogenesis. Front. Plant. Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Mine, I.; Menzel, D.; Okuda, K. Morphogenesis in giant-celled algae. Int. Rev. Cell Mol. Biol. 2008, 266, 37–83. [Google Scholar] [CrossRef]
- Cocquyt, E.; Verbruggen, H.; Leliaert, F.; De Clerck, O. Evolution and Cytological Diversification of the Green Seaweeds (Ulvophyceae). Mol. Biol. Evol. 2010, 27, 2052–2061. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.L.; You, S.G. Sulfated polysaccharides from green seaweeds. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 941–953. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, C.; Shao, Q.; Yang, Z.; Zhang, X.; Xu, X.; Hassan, M. Determination of water content in corn stover silage using near-infrared spectroscopy. Int. J. Agric. Biol. Eng. 2019, 12, 143–148. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Vejby Nielsen, B.; Harvey, P. A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Cao, Y.; Ma, Z.; Shao, Q. Optimization of liquid ammonia pretreatment conditions for maximizing sugar release from giant reed (Arundo donax L.). Biomass Bioenerg. 2017, 98, 61–69. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvares, C.; Twari, B.K.; O’Donnell, C.P. Extraction of biomolecules from seaweeds. In Seaweed Sustainability—Food and Non-Food Applications; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Jose, CA, USA, 2015; Volume 1, pp. 243–269. [Google Scholar]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.M.N.; Welti-Chanes, J. State-of-the-Art Extraction Methodologies for Bioactive Compounds from Algal Biome to Meet Bio-Economy Challenges and Opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiroma, R.; Uechi, S.; Taira, T.; Ishihara, M.; Tawata, S.; Tako, M. Isolation and Characterization of Fucoidan from Hizikia fusiformis (Hijiki). J. Appl. Glycosci. 2003, 50, 361–365. [Google Scholar] [CrossRef]
- Kim, W.-J.; Kim, S.-M.; Kim, H.G.; Oh, H.-R.; Lee, K.-B.; Lee, Y.-K.; Park, Y.-I. Purification and Anticoagulant Activity of a Fucoidan from Korean Undaria pinnatifida Sporophyll. Algae 2007, 22, 247–252. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process. Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-C. Extraction and characterization of fucoidan from six brown macroalgae. J. Mar. Sci. Technol. 2016, 24, 319–328. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Wei, X.L.; Sun, Z.L.; Wang, C.Y. Extraction, Fractionation, and Chemical Characterisation of Fucoidans from the Brown Seaweed Sargassum pallidum. Czech. J. Food Sci. 2016, 34, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Khalil, H.; Lai, T.; Tye, Y.; Rizal, S.; Chong, E.; Yap, S.; Hamzah, A.; Fazita, M.; Paridah, M. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Wang, S.; Qian, G.; Zhu, Q.; Liu, Y.; Wang, W. Optimization of Ultrasonic-assisted Extraction Technology of Sargassum fusiforme Polysaccharides and Evaluation of Their Antioxidant Activity. Food Sci. Technol. Res. 2013, 19, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Rafiquzzaman, S.M.; Rahman, A.; Kong, I.-S. Ultrasonic-Assisted Extraction of Carrageenan. Seaweed Polysacch. 2017, 75–81. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohyd. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohyd. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lakmal, H.H.C.; Lee, J.H.; Jeon, Y.J. Enzyme-Assisted Extraction of a Marine Algal Polysaccharide, Fucoidan and Bioactivities. In Polysaccharides - Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Cham, Switzerland, 2015; pp. 1065–1077. [Google Scholar] [CrossRef]
- Aðalbjörnsson, B.V.; Jónsdóttir, R. Enzyme-Enhanced Extraction of Antioxidant Ingredients from Algae. In Natural Products from Marine Algae—Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; pp. 145–150. [Google Scholar]
- Habeebullah, S.F.K.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghunaim, A.; Al-Yamani, F. Enzyme-assisted extraction of bioactive compounds from brown seaweeds and characterization. J. Appl. Phycol. 2019. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extracted of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łeska, B.; Messayasz, B.; Wilk, R.; Schroeder, G.; Dobrzy’nska-Inger, A.; et al. Supercriticalfluid extraction of algae enhances levels ofbiologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Martinez, J.; Martinez-Corre, H.A. Extraction of bioactive compounds from peach palm pulp (Bactris gasipaes) using supercritical CO2. J. Supercrit. Fluid. 2014, 93, 2–6. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohyd. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Rani, V.; Shakila, R.J.; Jawahar, P.; Srinivasan, A. Influence of Species, Geographic Location, Seasonal Variation and Extraction Method on the Fucoidan Yield of the Brown Seaweeds of Gulf of Mannar, India. Indian J. Pharm. Sci. 2017, 79, 65–71. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: an update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Kimica. About Alginate—Manufacturing Process. Available online: https://kimica-algin.com/alginate/process/ (accessed on 10 December 2019).
- McHugh, D.J. Alginate. In A Guide to the Seaweed Industry; FAO: Rome, Italy, 2003. [Google Scholar]
- Mazumder, A.; Løvstad Holdt, S.; De Francisci, D.; Alvarado-Morales, M.; Mishra, H.N.; Angelidaki, I. Extraction of alginate from Sargassum muticum: process optimization and study of its functional activities. J. Appl. Phycol. 2016. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, 3707–3714. [Google Scholar] [CrossRef] [Green Version]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S. Ultrasound-assisted extraction of sulfated polysaccharide from Nizamuddinia zanardinii: Process optimization, structural characterization, and biological properties. J. Food Process. Eng. 2018, 42, 1–13. [Google Scholar] [CrossRef]
- Hmelkov, A.B.; Zvyagintseva, T.N.; Shevchenko, N.M.; Rasin, A.B.; Ermakova, S.P. Ultrasound-assisted extraction of polysaccharides from brown alga Fucus evanescens. Structure and biological activity of the new fucoidan fractions. J. Appl. Phycol. 2018, 30, 2039–2046. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 2019, 31, 1391–1402. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kim, E.-K.; Park, J.-S.; Yoon, H.-D.; Kim, K.-R.; Ahn, C.-B. Antioxidant activity of enzymatic extracts from the brown seaweed Undaria pinnatifida by electron spin resonance spectroscopy. LWT-Food Sci. Technol. 2009, 42, 874–878. [Google Scholar] [CrossRef]
- Men’shova, R.V.; Lepeshkin, F.D.; Ermakova, S.P.; Pokrovskii, O.I.; Zvyagintseva, T.N. Effect of pretreatment conditions of brown algae by supercritical fluids on yield and structural characteristics of fucoidans. Chem. Nat. Compd. 2013, 48, 923–926. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; Lefèbvre D’Hellencourt, C.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohyd. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Jin, Y.; Zhang, W.; Liu, Y. Development of an eco-friendly agar extraction technique from the red seaweed Gracilaria lemaneiformis. Bioresour. Technol. 2008, 99, 3301–3305. [Google Scholar] [CrossRef]
- Luz Arvizu-Higuera, D.; Rodriguez-Montesinos, Y.E.; Murillo-Alvarez, J.I.; Munoz-Ochoa, M.; Hernandez-Carmona, G. Effect of alkali treatment time and extraction time on agar from Gracilaria vermiculophylla. In Nineteenth International Seaweed Symposium; Borowitzka, M.A., Critchley, A.T., Kraan, S., Peters, A., Sjøtun, K., Notoya, M., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 20, pp. 65–69. [Google Scholar]
- Distantina, S.; Wiratni, W.; Fahrurrozi, M.; Rochmadi, R. Carrageenan properties extracted from Eucheuma cottonii, Indonesia. World Acad. Sci. Eng. Technol. 2011, 78, 738–742. [Google Scholar]
- Tuvikene, R.; Truus, K.; Vaher, M.; Kailas, T.; Martin, G.; Kersen, P. Extraction and quantification of hybrid carrageenans from the biomass of the red algae Furcellaria lumbricalis and Coccotylus truncatus. Proc. Estonian Acad. Sci. Chem. 2006, 55, 40–53. [Google Scholar]
- Blanco-Pascual, N.; Alemán, A.; Gómez-Guillén, M.C.; Montero, M.P. Enzyme-assisted extraction of κ/ι-hybrid carrageenan from Mastocarpus stellatus for obtaining bioactive ingredients and their application for edible active film development. Food Funct. 2014, 5, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustapha, S.; Chandar, H.; Abidin, Z.Z.; Saghravani, R.; Harun, M.Y. Production of semi-refined carrageenan from Eucheuma cotonii. J. Sci. Ind. Res. 2011, 70, 865–870. [Google Scholar]
- Freile-Pelegrín, Y.; Robledo, D. Carrageenan of Eucheuma isiforme (Solieriaceae, Rhodophyta) from Nicaragua. J. Appl. Phycol. 2008, 20, 537–541. [Google Scholar] [CrossRef]
- Webber, V.; Matos de Carvalho, S.; Ogliari, P.J.; Hayashi, L.; Barreto, P.L.M. Optimization of the extraction of carrageenan from Kappaphycus alvarezii using response surface methodology. Food Sci. Technol. 2012, 32, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Bono, A.; Anisuzzaman, S.M.; Ding, O.W. Effect of process conditions on the gel viscosity and gel strength of semi-refined carrageenan (SRC) produced from seaweed (Kappaphycus alvarezii). J. King Saud Univ. Sci. 2014, 26, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Carmona, G.; Freile-Pelegrín, Y.; Hernández-Garibay, E. Conventional and alternative technologies for the extraction of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 475–516. [Google Scholar] [CrossRef]
- Heriyanto, H.; Kustiningsih, I.; Sari, D.K. The effect of temperature and time of extraction on the quality of Semi Refined Carrageenan (SRC). MATEC Web Conf. 2018, 154, 01034. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.V.; Bekheet, I.A. Preparation of agar-agar from the red seaweed Pterocladia capillacea off the coast of Alexandria, Egypt. Appl. Environ. Microbiol. 1976, 32, 479–482. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Read Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [Green Version]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumer. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mišurcová, L.; Machů, L.; Orsavová, J. Seaweed minerals as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wanibuchi, H.; Morimura, K.; Iwai, S.; Yoshida, K.; Endo, G.; Nakae, D.; Fukushima, S. Carcinogenicity of dimethylarsinic acid in male F344 rats and genetic alterations in induced urinary bladder tumors. Carcinogenesis 2002, 23, 1387–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Konishi, Y.; Matsuda, T.; Murai, T.; Shibata, M.A.; Matsui-Yuasa, I.; Otani, S.; Kuroda, K.; Endo, G.; Fukushima, S. Cancer induction by an organic arsenic compound, dimethylarsinic acid (cacodylic acid), in F344/DuCrj rats after pretreatment with five carcinogens. Cancer Res. 1995, 55, 1271–1276. [Google Scholar]
- van Weelden, G.; Bobinski, M.; Okła, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2018, 17, 32. [Google Scholar] [CrossRef] [Green Version]
- Helbert, W. Marine Polysaccharide Sulfatases. Front. Mar. Sci. 2017, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hanson, S.R.; Best, M.D.; Wong, C.-H. Sulfatases: Structure, Mechanism, Biological Activity, Inhibition, and Synthetic Utility. Angew. Chem. Int. Ed. Engl. 2004, 43, 5736–5763. [Google Scholar] [CrossRef]
- Ertesvåg, H. Alginate-modifying enzymes: biological roles and biotechnological uses. Front Microbiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Becker, S.; Scheffel, A.; Polz, M.F.; Hehemann, J.-H. Accurate Quantification of Laminarin in Marine Organic Matter with Enzymes from Marine Microbes. Appl. Environ. Microbiol 2017, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williams, D.L.; Martinez-Pomares, L.; Gordon, S. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y.C. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Otaka, K.; Maoka, T.; Hidaka, K.; Ishikima, S.; Oda, M.; Ohnishi, M. Structure of β-Glucan Oligomer from Laminarin and Its Effect on Human Monocytes to Inhibit the Proliferation of U937 Cells. Biosci. Biotechnol. Biochem. 2005, 69, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Dobruchowska, J.M.; Jonsson, J.O.; Fridjonsson, O.H.; Aevarsson, A.; Kristjansson, J.K.; Altenbuchner, J.; Watzlawick, H.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P.; et al. Modification of linear (β1→3)-linked gluco-oligosaccharides with a novel recombinant β-glucosyltransferase (trans-β-glucosidase) enzyme from Bradyrhizobium diazoefficiens. Glycobiology 2016, 26, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Jonsson-Wheat, J.O.; Hreggvidsson, G.O.; Fridjonsson, O.H.; Dobruchowska, J.M.; Kamerling, J.P. Glucan Branching Enzymes and Their Methods of Use. US20160265013A1, 26 March 2014. [Google Scholar]
- Hreggviðsson, G.H.; Dobruchowska, J.M.; Fridjonsson, O.H.; Jonsson, J.O.; Gerwig, G.J.; Aevarsson, A.; Kristjansson, J.K.; Curti, D.; Redgwell, R.R.; Hansen, C.-E.; et al. Exploring novel non-Leloir β-glucosyltransferases from proteobacteria for modifying linear (β1 → 3)-linked gluco-oligosaccharide chains. Glycobiology 2011, 21, 304–328. [Google Scholar] [CrossRef]
- Michel, G.; Czjzek, M. Polysaccharide-degrading enzymes from marine bacteria. In Marine Enzymes for Biocatalysis—Sources, Biocatalytic Characteristics and Bioprocesses of Marine Enzymes; Trincone, A., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 429–464. [Google Scholar] [CrossRef]
- Barbeyron, T.; Henrissat, B.; Kloareg, B. The gene encoding the kappa-carrageenase of Alteromonas carrageenovora is related to β-1,3-1,4-glucanases. Gene 1994, 139, 105–109. [Google Scholar] [CrossRef]
- Barbeyron, T.; Gerard, A.; Potin, P.; Henrissat, B.; Kloareg, B. The kappa-carrageenase of the marine bacterium Cytophaga drobachiensis. Structural and phylogenetic relationships within family-16 glycoside hydrolases. Mol. Biol. Evol. 1998, 15, 528–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, Y.; Hatada, Y. A Novel Enzyme, λ-Carrageenase, Isolated from a Deep-Sea Bacterium. J. Biochem. 2006, 140, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.T.; Kim, S.M. Agarase: review of major sources, categories, purification method, enzyme characteristics and applications. Mar. Drugs 2010, 8, 200–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, Y.; Hatada, Y.; Miyazaki, M.; Nogi, Y.; Ito, S.; Horikoshi, K. Purification and Characterization of a Novel α-Agarase from a Thalassomonas sp. Curr. Microbiol. 2005, 50, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Rochas, C.; Potin, P.; Kloareg, B. NMR spectroscopic investigation of agarose oligomers produced by an α-agarase. Carbohyd. Res. 1994, 253, 69–77. [Google Scholar] [CrossRef]
- Konasani, V.R.; Jin, C.; Karlsson, N.G.; Albers, E. A novel ulvan lyase family with broad-spectrum activity from the ulvan utilisation loci of Formosa agariphila KMM 3901. Sci. Rep. 2018, 8, 14713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.D.; Pati, M.; Nayak, L. Uses of Seaweed and Its Application to Human Welfare: A Review. Int. J. Pharm. Pharm. Sci. 2016, 8, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2015, 212. [Google Scholar] [CrossRef]
- Delaney, A.; Frangoudes, K.; Ii, S.A. Society and Seaweed: Understanding the Past and Present. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 7–40. [Google Scholar] [CrossRef]
- Mouritsen, O.G. Seaweeds: Edible, Available & Sustainable; University of Chicago Press Chicago & London: Chicago, IL, USA, 2013. [Google Scholar]
- Hamid, N.; Ma, Q.; Boulom, S.; Liu, T.; Zheng, Z.; Balbas, J.; Robertson, J. Seaweed minor constituents. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 193–242. [Google Scholar]
- Venkatraman, K.L.; Mehta, A. Health Benefits and Pharmacological Effects of Porphyra Species. Plant. Food Hum. Nutr. 2019, 74, 10–17. [Google Scholar] [CrossRef]
- van der Weele, C.; Feindt, P.; van der Goot, A.J.; van Mierlo, B.; van Boekel, M. Meat alternatives: An integrative comparison. Trends Food Sci. Technol. 2019, 88, 505–512. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquacult. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Shiroma, R.; Konishi, T.; Uechi, S.; Tako, M. Structural Study of Fucoidan from the Brown Seaweed Hizikia fusiformis. Food Sci. Technol. Res. 2008, 14, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Kadena, K.; Tomori, M.; Iha, M.; Nagamine, T. Absorption Study of Mozuku Fucoidan in Japanese Volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.-Y.; Hwang, P.-A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Trans. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and Cancer: A Multifunctional Molecule with Anti-Tumor Potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Pongsawatmanit, R.; Ikeda, S.; Miyawaki, O. Effect of temperature on viscoelastic properties of aqueous alginate solutions. Thai J. Agric. Sci. 1998, 31, 583–593. [Google Scholar]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Jiang, J.; Zhao, L.; Zhang, J.; Wang, F. Applications of Alginate as a Functional Food Ingredient. In Biopolymers for Food Design; Grumezescu, A., Butu, A., Eds.; Academic Press: Cambridge, UK, 2018; pp. 409–429. [Google Scholar]
- Jensen, M.G.; Kristensen, M.; Astrup, A. Effect of alginate supplementation on weight loss in obese subjects completing a 12-wk energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Houghton, D.; Wilcox, M.D.; Brownlee, I.A.; Chater, P.I.; Seal, C.J.; Pearson, J.P. Acceptability of alginate enriched bread and its effect on fat digestion in humans. Food Hydrocoll. 2019, 93, 395–401. [Google Scholar] [CrossRef]
- Odunsi, S.T.; Vázquez-Roque, M.I.; Camilleri, M.; Papathanasopoulos, A.; Clark, M.M.; Wodrich, L.; Lempke, M.; McKinzie, S.; Ryks, M.; Burton, D.; et al. Effect of Alginate on Satiation, Appetite, Gastric Function, and Selected Gut Satiety Hormones in Overweight and Obesity. Obesity 2010, 18, 1579–1584. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. β-Glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin Elicits Defense Responses in Grapevine and Induces Protection Against Botrytis cinerea and Plasmopara viticola. Mol. Plant. Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- UPL. Improve Overall Plant Health with Vacciplant®. Available online: https://us.uplonline.com/product-details/vacciplant (accessed on 11 December 2019).
- Seong, H.; Bae, J.-H.; Seo, J.S.; Kim, S.-A.; Kim, T.-J.; Han, N.S. Comparative analysis of prebiotic effects of seaweed polysaccharides laminaran, porphyran, and ulvan using in vitro human fecal fermentation. J. Funct. Foods 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Devillé, C.; Gharbi, M.; Dandrifosse, G.; Peulen, O. Study on the effects of laminaran, a polysaccharide from seaweed, on gut characteristics. J. Sci. Food Agric. 2007, 87, 1717–1725. [Google Scholar] [CrossRef]
- Zekovic, D.B.; Kwiatkowski, S.; Vrvic, M.M.; Jakovljevic, D.; Moran, C.A. Natural and modified (1→3)-β-D-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 2005, 25, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Dalonso, N.; Goldman, G.H.; Gernm, R.M. β-(1→3),(1→6)-Glucans: Medicinal activities, characterization, biosynthesis and new horizons. Appl. Microbiol. Biotechnol. 2015, 99, 7893–7906. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Kim, I.-H.; Kim, J.; Nam, T.-J. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. Int. J. Mol. Med. 2013, 32, 291–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfim-Mendonça, P.S.; Capoci, I.; Tobaldini-Valerio, F.K.; Negri, M.; Svidzinski, T. Overview of β-Glucans from Laminaria spp.: Immunomodulation properties and applications on biologic models. Int. J. Mol. Sci. 2017, 18, 1629. [Google Scholar] [CrossRef] [Green Version]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13. [Google Scholar] [CrossRef] [Green Version]
- Distantina, S.; Rochmadi, R.; Fahrurrozi, M.; Wiratni, W. Synthesis of Hydrogel Film Based on Carrageenan Extracted from Kappaphycus alvarezii. Mod. Appl. Sci. 2013, 7, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y. Seaweed Hydrocolloids as Thickening, Gelling, and Emulsifying Agents in Functional Food Products. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: San Diego, CA, USA, 2018; pp. 135–152. [Google Scholar]
- Pangestuti, R.; Kim, S.-K. Biological Activities of Carrageenan. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: San Diego, CA, USA, 2014; Volume 72, pp. 113–124. [Google Scholar]
- Carlucci, M.J.; Scolaro, L.A.; Noseda, M.D.; Cerezo, A.S.; Damonte, E.B. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antivir. Res. 2004, 64, 137–141. [Google Scholar] [CrossRef]
- Eccles, R.; Meier, C.; Jawad, M.; Weinmüllner, R.; Grassauer, A.; Prieschl-Grassauer, E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: A randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir. Res. 2010, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panlasigui, L.; Baello, O.; Dimatangal, J.; Dumelod, B. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia. Pac. J. Clin. Nutr. 2003, 12, 209–214. [Google Scholar] [PubMed]
- Haijin, M.; Xiaolu, J.; Huashi, G. A κ-carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity. J. Appl. Phycol. 2003, 15, 297–303. [Google Scholar] [CrossRef]
- Zhou, G.; Sheng, W.; Yao, W.; Wang, C. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Barabanova, A.O.; Homenko, V.A.; Solov’eva, T.F.; Bogdanovich, R.N.; Yermak, I.M. In Vitro and Ex Vivo Studies of Antioxidant Activity of Carrageenans, Sulfated Polysaccharides from Red Algae. Bull. Exp. Biol. Med. 2011, 150, 426–428. [Google Scholar] [CrossRef]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Jiang, X.; Aubree, E.; Boulenguer, P.; Critchley, A.T. Preparation and In Vivo. Antitumor Activity of κ-Carrageenan Oligosaccharides. Pharm. Biol. 2006, 44, 646–650. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Zhang, W.; Li, X.; Lü, X.; Li, N.; Gao, X.; Song, J. Preparation and in vitro antioxidant activity of κ-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives. Carbohyd. Res. 2005, 340, 685–692. [Google Scholar] [CrossRef]
- Xu, L.; Yao, Z.; Wu, H.; Wang, F.; Zhang, S. The immune regulation of κ-carrageenan oligosaccharide and its desulfated derivatives on LPS-activated microglial cells. Neurochem. Int. 2012, 61, 689–696. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Saxena, A. Bacterial carrageenases: An overview of production and biotechnological applications. 3 Biotech. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohyd. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, G.; Carrow, J.K.; Thakur, T.; Xavier, J.R.; Parani, M.; Bayless, K.J.; Gaharwar, A.K. Nanoengineered injectable hydrogels for wound healing application. Acta Biomat. 2018, 70, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, M.; Cui, S.; Wang, Q. Polysaccharide Gums: Structures, Functional Properties, and Applications. In Food Carbohydrates: Chemistry, Physical Properties and Applications, 1st ed.; Cui, S.W., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Marcus, J.B. Food Science Basics: Healthy Cooking and Baking Demystified: The Science behind Healthy Foods, Cooking and Baking. In Culinary Nutrition; Marcus, J.B., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 51–97. [Google Scholar]
- Armisén, R.; Gaiatas, F. Agar. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 82–107. [Google Scholar]
- Serwer, P. Agarose gels: Properties and use for electrophoresis. Electrophoresis 1983, 4, 375–382. [Google Scholar] [CrossRef]
- Armisén, R. Agar and agarose biotechnological applications. Hydrobiologia 1991, 221, 157–166. [Google Scholar] [CrossRef]
- Ream, J.A.; Lewis, L.K.; Lewis, K.A. Horizontal Agarose Gel Mobility Shift Assay for Protein-RNA Complexes. In Electrophoretic Separation of Proteins: Methods and Protocols; Kurien, B.T., Scofield, R.H., Eds.; Springer: New York, NY, USA, 2019; pp. 363–370. [Google Scholar]
- Aurelien, F.; Jon, C.; Steffen, L.; Esther, K.; Simon, T.; Maziar, M.; Ralf, T.; Shastri, V.P. Polysaccharide hydrogels with tunable stiffness and provasculogenic properties via α-helix to β-sheet switch in secondary structure. Proc. Natl. Acad. Sci. USA 2013, 110. [Google Scholar] [CrossRef] [Green Version]
- Forget, A.; Pique, R.A.; Ahmadi, V.; Lu¨deke, S.; Shastri, V.P. Mechanically Tailored Agarose Hydrogels through Molecular Alloying with β-Sheet Polysaccharides. Macromol. Rapid Commun. 2015, 36, 196–203. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; You, S.G. Molecular characteristics of sulfated polysaccharides from Monostroma nitidum and their in vitro anticancer and immunomodulatory activities. Int. J. Biol. Macromol. 2011, 48, 311–318. [Google Scholar] [CrossRef]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Huang, L.; Liu, X.; Liu, D.; Zhang, Q.; Liu, S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Mao, W.J.; Fang, F.; Li, H.Y.; Qi, X.H.; Sun, H.H.; Chen, Y.; Guo, S.D. Heparinoid-active two sulfated polysaccharides isolated from marine green algae Monostroma nitidum. Carbohydr. Polym. 2008, 74, 834–839. [Google Scholar] [CrossRef]


| Polysaccharide | Structure 1 | |
|---|---|---|
| Alginate |  β-1,4-d-mannuronic acid (M) and α-1,4-l-guluronic acid (G) residues forming GG, MM and M/G blocks | |
| Fucoidan |  Alternating 1,3- and 1,4-linked α- l-fucopyranose |  α-1,3-l-fucopyranose |
| Laminarin |  β-1,3-d-glucopyranose backbone with branching β-1,6-d-glucopyranose unit | |
| Carrageenan |  µ-carrageenan: R1 = SO3−, R2 = R3 = H ν-carrageenan: R1 = R3 = SO3−, R2 = H λ-carrageenan: R1 = H, R2 = R3 = SO3− Alternating α-1,4-d-galactopyranose and β-1,3-d-galactopyranose |  κ-carrageenan: R1 = SO3−, R2 = R3 = H ι-carrageenan: R1 = R3 = SO3−, R2 = H θ-carrageenan: R1 = H, R2 = R3 = SO3 Alternating β-1,3-d-galactopyranose and 3,6-anhydro-α-d-galactopyranose |
| Agar |  |  |
| R = H or side chain substituents e.g., sulfate ester, methoxyl ether or pyruvic acid | ||
| Alternating β-1,3-d-galactopyranose and 3,6-anhydro-α-1,4-l-galactopyranose | Alternating β-1,3-d-galactopyranose and α-1,4-l-galactopyranose | |
| Ulvan |  Alternating β-1,4-d-glucuronic acid and α-1,4-l-rhamnopyranose (from Ulva rigida) |  Alternating α-1,4-l-iduronic acid and α-1,4-l-rhamnopyranose (from Ulva armoricana) |
 Alternating β-1,4-d-xylanopyranose and α-1,4-l-rhamnopyranose (as shown in Enteromorpha sp) | ||
| Extraction Method | Principle | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Conventional chemical extraction | Different procedures of acid or alkaline extraction and hot or cold water extraction. Acidic or alkaline conditions are usually applied to facilitate extraction, as hydrogen ions (H+) and hydroxyl ions (OH−) interfere with hydrogen linkages between polysaccharides. The conventional extraction procedures rely on the solubility properties of target compounds and are often preceded by pretreatment steps where lipids, pigments, proteins and other impurities are removed by solvents. | Well established methods. | Long extraction time. High consumption of energy and water. Alcohol precipitation and recovery are costly. Chemical solvents may have health hazards. Acids and bases can cause degradation of the target polysaccharide to compounds of smaller molecular size. | [56,57,58,59,60,61] |
| Ultrasound assisted extraction (UAE) | UAE acts by exposing the biomass to sound waves of high frequencies larger than 20 kHz. Strong ultrasound fields cause the implosion of vapor bubbles in liquids formed under high pressure conditions. Implosion of these bubbles in near proximity to liquid-solid borders, such as cell walls, subject these solid surfaces to strong forces resulting in cell breakdown. UAE can be performed at low temperatures which enable the extraction of thermosensitive target compounds. | Short extraction time. Higher yields of extracted bioactives. Simple method to operate. Operation at low temperatures. Relatively low amounts of solvent are required. | Degradation and structural changes in the structure of polysaccharides. | [54,62,63] |
| Microwave assisted extraction (MAE) | MAE utilizes non-ionizing electromagnetic radiation with frequencies between 300 MHz and 300 GHz which cause the disruption of hydrogen bonds and migration of dissolved ions. This enables the solvent to enter the cell matrix and facilitate the withdrawal of compounds of interest. Variables such as temperature, pressure, time and algae/water ratio can be altered to optimize the yield of the desired product. | Short extraction time. Relatively low amounts of solvent are required. Higher quality of product. | Lower yield is achieved due to degradation. | [54,61,64,65] |
| Enzyme assisted extraction (EAE) | EAE operates by using enzymes for degradation of the algal cell wall, thereby releasing target compounds. Critical parameters, including pH, temperature and treatment time, should be optimized for specific enzymes to maximize the extraction result. Enzymes catalyzing degradative reactions of cell wall structure compounds like cellulose, β-glucan and hemicellulose are usually used to facilitate the extraction of target molecules. | Relatively low amounts of solvent are required. Relatively low-cost technique. Disruption of cell wall components is enzymatically performed. Original efficacy of bioactives is preserved to a high degree. Potential of a higher yield of the target compound. The mild conditions applied to the sample during EAE are advantageous when isolating sensitive bioactive compounds. | Extraction yield is dependent on optimum treatment time, pH and temperature conditions of enzymes. Target compounds risk being degraded by non-specific enzymes. Extraction efficiency is dependent on enzyme properties. | [54,66,67,68,69] |
| Supercritical fluid extraction (SFE) | A supercritical fluid is a substance at a temperature and pressure above its critical point, where gas and liquid phases are indistinct. Altering the two parameters can change the solubility of the fluid. Carbon dioxide is commonly used as its critical point is relatively low and thus requires less energy input to become supercritical, compared to substances with higher critical points. Supercritical fluids are characterized for their low viscosity and high diffusivity which gives them better transport properties than liquids. SFE is considered an environmentally friendly process as it does not require the use of solvents. However, procurement costs are high in relation to other extraction methods. This concept is therefore predominantly employed to extract highly valuable compounds. | Use of non-toxic and non-flammable solvent. Supercritical CO2 is inexplosive, readily available, and can be removed easily from the final extract. Does not cause degradation and structural disruptions in bioactive compounds. | High pressure is needed to maintain the solvent in critical state, which can have negative effect on compounds. Relatively high procurement costs. | [54,70,71,72] |
| Polysaccharide | Yield 1 | Species | Method | Ref. |
|---|---|---|---|---|
| Fucoidan | 1.63% | Padina tetrastromatica | Conventional extraction (HCl) | [74] |
| 9.46% | P. tetrastromatica | Conventional extraction (hot water) | [74] | |
| 3.51% | Nizamuddinia zanardinii | UAE | [80] | |
| 4.44% (4.7%, CaCl2) | Fucus evanescens | UAE | [81] | |
| 18.22% | Fucus vesiculosus | MAE | [64] | |
| 16.08% (20.98%, HCl) | Ascophyllum nodosum | MAE | [65] | |
| 5.58%, Alcalase 4.80%, Cellulase 4.36%, Flavourzyme 4.28%, Viscozyme (5.20%, hot water) | N. zanardinii | EAE | [82] | |
| 1.5 ± 0.3% | U. pinnatifida | EAE | [83] | |
| 3.02% (5.11%, EtOH) | F. evanescens | SFE | [84] | |
| 1.26% (1.35%, SFE-EtOH) (1.28%, EtOH) | S. japonica | SFE | [84] | |
| 0.57% (0.55%, SFE-EtOH) (0.65%, EtOH) | Sargassum oligocystum | SFE | [84] | |
| Alginate | 51.8% | L. digitata | Conventional extraction (HCl) | [79] |
| 13.47% | Sargassum muticum | Conventional extraction (HCl) | [78] | |
| 54% 2 | Sargassum binderi | UAE | [85] | |
| 23.6 ± 1.2% | U. pinnatifida | EAE | [83] | |
| Laminarin | 6.0 ± 0.7% | S. latissima | Conventional extraction (HCl + EtOH) | [33] |
| 19 ± 2.6% | S. latissima | Conventional extraction (HCl + NaOH) | [33] | |
| 20% | S. latissima | Conventional extraction (hot H2SO4) | [33] | |
| 6.1 ± 1.6% | S. latissima | Conventional extraction (hot HCl) | [33] | |
| 3.2 ± 0.9% | U. pinnatifida | EAE | [83] | |
| Agar | 29.7 ± 1.9% Gel strength: 271 ± 38 g/cm2 | Gracilaria lemaneiformis | Conventional extraction (hot water) | [86] |
| 25.8 ± 2.9% (Gel strength: 1761 ± 35 g/cm2) | G. lemaneiformis | Conventional extraction (alkali pre-treatment with 5% NaOH + hot water) | [86] | |
| 25.4 ± 1.7% (Gel strength: 1913 ± 38 g/cm2) | G. lemaneiformis | Conventional extraction (alkali pre-treatment with 5% NaOH +Photobleaching + hot water) | [86] | |
| 29.7–34.6% (Gel strength: 72 g/cm2) | Gracilaria vermiculophylla | Conventional extraction (hot water) | [87] | |
| 15.03% (Gel strength: 1064 g/cm2) | G. vermiculophylla | Conventional extraction (pre-treatment with 7% NaOH + hot water) | [87] | |
| Carrageenan | 46.43% | E. cottonii | Conventional extraction (hot water) | [88] |
| 37.02% | E. cottonii | Conventional Extraction (KOH) | [88] | |
| 76.3% | Furcellaria lumbricalis Coccotylus truncates | Conventional extraction (hot water) | [89] | |
| 72.6% | F. lumbricalis C. truncatus | Conventional extraction (0.15 M NaOH) | [89] | |
| 55.3% | F. lumbricalis C. truncatus | Conventional extraction (0.15 M KOH) | [89] | |
| 50–55% (for both species) 3 | K. alvarezii Euchema denticulatum | UAE | [85] | |
| 28.65% | Mastocarpus stellatus | EAE | [90] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules 2020, 25, 930. https://doi.org/10.3390/molecules25040930
Jönsson M, Allahgholi L, Sardari RRR, Hreggviðsson GO, Nordberg Karlsson E. Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules. 2020; 25(4):930. https://doi.org/10.3390/molecules25040930
Chicago/Turabian StyleJönsson, Madeleine, Leila Allahgholi, Roya R.R. Sardari, Guðmundur O. Hreggviðsson, and Eva Nordberg Karlsson. 2020. "Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications" Molecules 25, no. 4: 930. https://doi.org/10.3390/molecules25040930
APA StyleJönsson, M., Allahgholi, L., Sardari, R. R. R., Hreggviðsson, G. O., & Nordberg Karlsson, E. (2020). Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules, 25(4), 930. https://doi.org/10.3390/molecules25040930