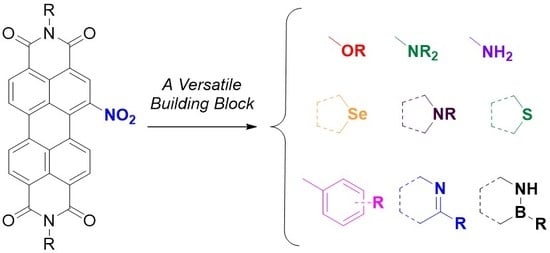
Nitro-Perylenediimide: An Emerging Building Block for the Synthesis of Functional Organic Materials
Abstract
:1. Introduction
2. Nitration vs. Bromination Conditions of Perylenediimide Derivatives
3. Reactivity of 1-NitroPerylenediimide
3.1. Nucleophilic Substitution of the Nitro Group
3.2. Access to Core-Extended Annulated PDI
3.3. Palladium-Catalyzed Cross-Coupling Reactions
4. Reduction of the Nitro Group and Functionalization of AminoPDI
4.1. Preparation 1-aminoPDI from 1-nitroPDI
4.2. Reactivity of 1-AminoPDI
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kardos, M. German Patent DE 276357, 1913.
- Langhals, H. Cyclic carboxylic imide structures as structure elements of high stability. Novel developments in perylene dye chemistry. Heterocycles 1995, 40, 477–500. [Google Scholar] [CrossRef] [Green Version]
- Zollinger, H. Color Chemistry, 3rd ed.; Wiley VCH: Weinheim, Germany, 2003. [Google Scholar]
- Herbst, W.; Hunger, K. Industrial Organic Pigments: Production, Properties, Applications, 3rd ed.; Wiley VWH: Weinheim, Germany, 2004. [Google Scholar]
- Loutfy, R.; Hor, A.; Kazmaier, P.; Tam, M. Layered organic photoconductive (OPC) devices incorporating N, N’-disubstituted diimide and bisarylimidazole derivatives of perylene-3,4,9,10-tetracarboxylic acid. J. Imaging Sci. 1989, 33, 151–159. [Google Scholar]
- Soh, N.; Ueda, T. Perylene bisimide as a versatile fluorescent tool for environmental and biological analysis: A review. Talanta 2011, 85, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Quante, H.; Geerts, Y.; Müllen, K. Synthesis of Soluble Perylenebisamidine Derivatives. Novel Long-Wavelength Absorbing and Fluorescent Dyes. Chem. Mater. 1997, 9, 495–500. [Google Scholar] [CrossRef]
- Sánchez, R.S.; Gras-Charles, R.; Bourdelande, J.L.; Guirado, G.; Hernando, J. Light- and Redox-Controlled Fluorescent Switch Based on a Perylenediimide–Dithienylethene Dyad. J. Phys. Chem. C 2012, 116, 7164–7172. [Google Scholar]
- Yu, Z.; Wu, Y.; Liao, Q.; Zhang, H.; Bai, S.; Li, H.; Xu, Z.; Sun, C.; Wang, X.; Yao, J.; et al. Self-Assembled Microdisk Lasers of Perylenediimides. J. Am. Chem. Soc. 2015, 137, 15105–15111. [Google Scholar] [CrossRef]
- Anthony, J.E. Small-Molecule, Nonfullerene Acceptors for Polymer Bulk Heterojunction Organic Photovoltaics. Chem. Mater. 2011, 23, 583–590. [Google Scholar] [CrossRef]
- Li, C.; Wonneberger, H. Perylene Imides for Organic Photovoltaics: Yesterday, Today, and Tomorrow. Adv. Mat. 2012, 24, 613–636. [Google Scholar] [CrossRef]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dyes Pigm. 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Fernández-Lázaro, F.; Zink-Lorre, N.; Sastre-Santos, Á. Perylenediimides as non-fullerene acceptors in bulk-heterojunction solar cells (BHJSCs). J. Mater. Chem. A 2016, 4, 9336–9346. [Google Scholar] [CrossRef]
- Wasielewski, M.R. Self-Assembly Strategies for Integrating Light Harvesting and Charge Separation in Artificial Photosynthetic Systems. Acc. Chem. Res. 2009, 42, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, M.P.; Niemczyk, M.P.; Svec, W.A.; Gosztola, D.; Gaines, G.L., III; Wasielewski, M.R. Picosecond optical switching based on biphotonic excitation of an electron donor-acceptor-donor molecule. Science 1992, 257, 63–65. [Google Scholar] [CrossRef]
- Prathapan, S.; Yang, S.I.; Seth, J.; Miller, M.A.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Synthesis and Excited-State Photodynamics of Perylene-Porphyrin Dyads. 1. Parallel Energy and Charge Transfer via a Diphenylethyne Linker. J. Phys. Chem. B 2001, 105, 8237–8248. [Google Scholar] [CrossRef]
- Serin, J.M.; Brousmiche, D.W.; Fréchet, J.M.J. Cascade energy transfer in a conformationally mobile multichromophoric dendrimer. Chem. Commun. 2002, 2605–2607. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S.; Ohkubo, K.; Ortiz, J.; Gutierrez, A.M.; Fernandez-Lazaro, F.; Sastre-Santos, A. Formation of a long-lived charge-separated state of a zinc phthalocyanine-perylenediimide dyad by complexation with magnesium ion. Chem. Commun. 2005, 3814–3816. [Google Scholar] [CrossRef]
- An, Z.; Odom, S.A.; Kelley, R.F.; Huang, C.; Zhang, X.; Barlow, S.; Padilha, L.A.; Fu, J.; Webster, S.; Hagan, D.J.; et al. Synthesis and Photophysical Properties of Donor- and Acceptor-Substituted 1,7-Bis(arylalkynyl)perylene-3,4:9,10-bis(dicarboximide)s. J. Phys. Chem. A 2009, 113, 5585–5593. [Google Scholar] [CrossRef]
- Shoaee, S.; An, Z.; Zhang, X.; Barlow, S.; Marder, S.R.; Duffy, W.; Heeney, M.; McCulloch, I.; Durrant, J.R. Charge photogeneration in polythiophene-perylene diimide blend films. Chem. Commun. 2009, 5445–5447. [Google Scholar] [CrossRef]
- Rocard, L.; Berezin, A.; De Leo, F.; Bonifazi, D. Templated Chromophore Assembly by Dynamic Covalent Bonds. Angew. Chem. Int. Ed. 2015, 54, 15739–15743. [Google Scholar] [CrossRef]
- Rocard, L.; Wragg, D.; Jobbins, S.A.; Luciani, L.; Wouters, J.; Leoni, S.; Bonifazi, D. Templated Chromophore Assembly on Peptide Scaffolds: A Structural Evolution. Chem. Eur. J. 2018, 24, 16136–16148. [Google Scholar] [CrossRef]
- Yukruk, F.; Dogan, A.L.; Canpinar, H.; Guc, D.; Akkaya, E.U. Water-Soluble Green Perylenediimide (PDI) Dyes as Potential Sensitizers for Photodynamic Therapy. Org. Lett. 2005, 7, 2885–2887. [Google Scholar] [CrossRef]
- Sun, M.; Müllen, K.; Yin, M. Water-soluble perylenediimides: Design concepts and biological applications. Chem. Soc. Rev. 2016, 45, 1513–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncali, J. Synthetic principles for bandgap control in linear π-conjugated systems. Chem. Rev. 1997, 97, 173–205. [Google Scholar] [CrossRef] [PubMed]
- Roncali, J. Molecular Engineering of the Band Gap of π-Conjugated Systems: Facing Technological Applications. Macromol. Rapid Commun. 2007, 28, 1761–1775. [Google Scholar] [CrossRef]
- Miletić, T.; Fermi, A.; Orfanos, I.; Avramopoulos, A.; De Leo, F.; Demitri, N.; Bergamini, G.; Ceroni, P.; Papadopoulos, M.G.; Couris, S.; et al. Tailoring Colors by O Annulation of Polycyclic Aromatic Hydrocarbons. Chem. Eur. J. 2017, 23, 2363–2378. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic Acid Diimides: Synthesis, Physical Properties, and Use in Organic Electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef]
- Nowak-Krol, A.; Würthner, F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-Y.; Chang, C.-W. Highly soluble monoamino-substituted perylene tetracarboxylic dianhydrides: Synthesis, optical and electrochemical properties. Int. J. Mol. Sci. 2014, 15, 22642–22660. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Chang, C.-W.; Chen, K.-Y. 1,6- and 1,7-regioisomers of dinitro- and diamino-substituted perylene bisimides: Synthesis, photophysical and electrochemical properties. Tetrahedron Lett. 2014, 55, 884–888. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Zhang, A.; Wang, W.; Cui, G.; Zhao, J.; Shi, Z.; Tang, B. Efficient energy-level modification of novel pyran-annulated perylene diimides for photocatalytic water splitting. Chem. Commun. 2017, 53, 6918–6921. [Google Scholar] [CrossRef] [Green Version]
- Rocard, L.; Hatych, D.; Chartier, T.; Cauchy, T.; Hudhomme, P. Original Suzuki–Miyaura Coupling Using Nitro Derivatives for the Synthesis of Perylenediimide-Based Multimers. Eur. J. Org. Chem. 2019, 2019, 7635–7643. [Google Scholar] [CrossRef]
- Gupta, R.K.; Shankar Rao, D.S.; Prasad, S.K.; Achalkumar, A.S. Columnar Self-Assembly of Electron-Deficient Dendronized Bay-Annulated Perylene Bisimides. Chem. Eur. J. 2018, 24, 3566–3575. [Google Scholar] [CrossRef] [PubMed]
- Goujon, A.; Rocard, L.; Cauchy, T.; Hudhomme, P. An Efficient Imine Photocyclization as an Alternative to the Pictet-Spengler Reaction for the Synthesis of AzaBenzannulated Perylenediimide Dyes. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Würthner, F. Bay-substituted perylene bisimides: Twisted fluorophores for supramolecular chemistry. Pure Appl. Chem. 2006, 78, 2341–2349. [Google Scholar] [CrossRef]
- Rajasingh, P.; Cohen, R.; Shirman, E.; Shimon, L.J.W.; Rybtchinski, B. Selective Bromination of Perylene Diimides under Mild Conditions. J. Org. Chem. 2007, 72, 5973–5979. [Google Scholar] [CrossRef]
- Langhals, H.; Kirner, S. Novel fluorescent dyes by the extension of the core of perylenetetracarboxylic bisimides. Eur. J. Org. Chem. 2000, 365–380. [Google Scholar] [CrossRef]
- Dominguez, C.; Baena, M.J.; Coco, S.; Espinet, P. Perylenecarboxydiimide-gold(I) organometallic dyes. Optical properties and Langmuir films. Dyes Pigm. 2017, 140, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-Y.; Chow, T.J. 1,7-Dinitroperylene bisimides: Facile synthesis and characterization as n-type organic semiconductors. Tetrahedron Lett. 2010, 51, 5959–5963. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Fang, T.-C.; Chang, M.-J. Synthesis, photophysical and electrochemical properties of 1-aminoperylene bisimides. Dyes Pigm. 2012, 92, 517–523. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wang, L.M.; Zou, G.; Zhang, L.; Zhang, G.J.; Cai, X.F.; Teng, M.S. Colorimetric and ratiometric fluorescent chemosensor for fluoride ion based on perylene diimide derivatives. Dyes Pigm. 2012, 94, 410–415. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhang, G.; Wu, Y.; Wu, S.; Yu, J.; Wang, L. A new colorimetric and fluorescent bifunctional probe for Cu2+ and F- ions based on perylene bisimide derivatives. Tetrahedron Lett. 2014, 55, 3218–3222. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Chen, K.-Y. Synthesis and optical properties of novel asymmetric perylene bisimides. J. Lumin. 2014, 149, 103–111. [Google Scholar] [CrossRef]
- Rosenne, S.; Grinvald, E.; Shirman, E.; Neeman, L.; Dutta, S.; Bar-Elli, O.; Ben-Zvi, R.; Oksenberg, E.; Milko, P.; Kalchenko, V.; et al. Self-Assembled Organic Nanocrystals with Strong Nonlinear Optical Response. Nano Lett. 2015, 15, 7232–7237. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Sun, D.; Zhong, C.; Liu, T.; Fan, B.; Huo, L.; Li, Y.; Jiang, W.; Choi, H.; Kim, T.; et al. High-Performance Solution-Processed Non-Fullerene Organic Solar Cells Based on Selenophene-Containing Perylene Bisimide Acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, K.; Bhargava, G.; Kumar, S. Self-assembled nanorods of bay functionalized perylenediimide: Cu2+ based ‘turn-on’ response for INH, complementary NOR/OR and TRANSFER logic functions and fluorosolvatochromism. J. Mater. Chem. C 2016, 4, 2488–2497. [Google Scholar] [CrossRef]
- Kumar, K.; Bhargava, G.; Kumar, S.; Singh, P. Dissymmetric Bay-Functionalized Perylenediimides. Synlett 2018, 29, 1693–1699. [Google Scholar]
- Singh, P.; Mittal, L.S.; Vanita, V.; Kumar, K.; Walia, A.; Bhargava, G.; Kumar, S. Self-assembled vesicle and rod-like aggregates of functionalized perylene diimide: Reaction-based near-IR intracellular fluorescent probe for selective detection of palladium. J. Mater. Chem. B 2016, 4, 3750–3759. [Google Scholar] [CrossRef]
- Hendsbee, A.D.; Sun, J.-P.; Law, W.K.; Yan, H.; Hill, I.G.; Spasyuk, D.M.; Welch, G.C. Synthesis, Self-Assembly, and Solar Cell Performance of N-Annulated Perylene Diimide Non-Fullerene Acceptors. Chem. Mater. 2016, 28, 7098–7109. [Google Scholar] [CrossRef]
- El-Berjawi, R.; Hudhomme, P. Synthesis of a perylenediimide-fullerene C60 dyad: A simple use of a nitro leaving group for a Suzuki-Miyaura coupling reaction. Dyes Pigm. 2018, 159, 551–556. [Google Scholar] [CrossRef]
- Hruzd, M.; Rocard, L.; Allain, M.; Hudhomme, P. Suzuki-Miyaura Coupling on Dinitro-bay Substituted Perylenediimide. 2020; Unpublished results; manuscript in preparation. [Google Scholar]
- Tsai, H.-Y.; Chang, C.-W.; Chen, K.-Y. 1,6- and 1,7-regioisomers of asymmetric and symmetric perylene bisimides: Synthesis, characterization and optical properties. Molecules 2014, 19, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Dubey, R.K.; Efimov, A.; Lemmetyinen, H. 1,7- And 1,6-Regioisomers of Diphenoxy and Dipyrrolidinyl Substituted Perylene Diimides: Synthesis, Separation, Characterization, and Comparison of Electrochemical and Optical Properties. Chem. Mater. 2011, 23, 778–788. [Google Scholar] [CrossRef]
- Kong, X.; Gao, J.; Ma, T.; Wang, M.; Zhang, A.; Shi, Z.; Wei, Y. Facile synthesis and replacement reactions of mono-substituted perylene bisimide dyes. Dyes Pigm. 2012, 95, 450–454. [Google Scholar] [CrossRef]
- Sun, D.; Meng, D.; Cai, Y.; Fan, B.; Li, Y.; Jiang, W.; Huo, L.; Sun, Y.; Wang, Z. Non-Fullerene-Acceptor-Based Bulk-Heterojunction Organic Solar Cells with Efficiency over 7%. J. Am. Chem. Soc. 2015, 137, 11156–11162. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Cieplechowicz, E.; Welsh, T.A.; Welch, G.C. A direct comparison of monomeric vs. dimeric and non-annulated vs. N-annulated perylene diimide electron acceptors for organic photovoltaics. New J. Chem. 2019, 43, 5187–5195. [Google Scholar] [CrossRef]
- Ma, Z.; Fu, H.; Meng, D.; Jiang, W.; Sun, Y.; Wang, Z. Isomeric N-Annulated Perylene Diimide Dimers for Organic Solar Cells. Chem. Asian J. 2018, 13, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Cann, J.R.; Cabanetos, C.; Welch, G.C. Synthesis of Molecular Dyads and Triads Based Upon N-Annulated Perylene Diimide Monomers and Dimers. Eur. J. Org. Chem. 2018, 2018, 6933–6943. [Google Scholar] [CrossRef]
- Chen, L.; Xia, P.; Du, T.; Deng, Y.; Xiao, Y. Catalyst-Free One-Pot Synthesis of Unsymmetrical Five- and Six-Membered Sulfur-Annulated Heterocyclic Perylene Diimides for Electron-Transporting Property. Org. Lett. 2019, 21, 5529–5532. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Z.; Zhang, A.; Li, J.; Wei, X.; Jiang, T.; Li, Y.; Wang, X. Self-assembly, optical and electrical properties of five membered O- or S-heterocyclic annulated perylene diimides. Dyes Pigm. 2016, 135, 41–48. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Zhou, Y.; Hao, P.; Shang, Q.; Wang, S.; Zhang, Y.; Li, D.; Yang, S.; Zhang, Q. Facile Syntheses, Characterization, and Physical Properties of Sulfur-Decorated Pyran-Annulated Perylene Diimides. Asian J. Org. Chem. 2018, 7, 702–706. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Nakayama, H.; Wei, Z.; Schneider, J.A.; Clark, K.; Lai, W.-Y.; Huang, W.; Labram, J.G.; de Alaniz, J.R.; et al. Multi-Sulfur-Annulated Fused Perylene Diimides for Organic Solar Cells with Low Open-Circuit Voltage Loss. ACS Appl. Energy Mater. 2019, 2, 3805–3814. [Google Scholar] [CrossRef]
- Yang, Y. Palladium-Catalyzed Cross-Coupling of Nitroarenes. Angew. Chem. Int. Ed. 2017, 56, 15802–15804. [Google Scholar] [CrossRef]
- Yadav, M.R.; Nagaoka, M.; Kashihara, M.; Zhong, R.-L.; Miyazaki, T.; Sakaki, S.; Nakao, Y. The Suzuki-Miyaura Coupling of Nitroarenes. J. Am. Chem. Soc. 2017, 139, 9423–9426. [Google Scholar] [CrossRef] [PubMed]
- Rocard, L.; Hudhomme, P. Recent developments in the Suzuki-Miyaura reaction using nitroarenes as electrophilic coupling reagents. Catalysts 2019, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Aivali, S.; Tsimpouki, L.; Anastasopoulos, C.; Kallitsis, J.K. Synthesis and optoelectronic characterization of perylene diimide-quinoline based small molecules. Molecules 2019, 24, 4406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.-Y.; Chen, K.-Y. 1,7-Diaminoperylene bisimides: Synthesis, optical and electrochemical properties. Dyes Pigm. 2013, 96, 319–327. [Google Scholar] [CrossRef]
- Hao, L.; Jiang, W.; Wang, Z. Integration of nitrogen into coronene bisimides. Tetrahedron 2012, 68, 9234–9239. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.; Li, J.; Cao, J.; Zhu, J.; Sun, X.W.; Zhang, Q. Synthesis, Characterization, Physical Properties, and OLED Application of Single BN-Fused Perylene Diimide. J. Org. Chem. 2015, 80, 196–203. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yu, J.; Zhang, G.; Cai, X.; Wu, Y.; Wang, L. A colorimetric and fluorescent sensor based on PBIs for palladium detection. Tetrahedron Lett. 2013, 54, 4019–4022. [Google Scholar] [CrossRef] [Green Version]
- Schulze, M.; Philipp, M.; Waigel, W.; Schmidt, D.; Wuerthner, F. Library of Azabenz-Annulated Core-Extended Perylene Derivatives with Diverse Substitution Patterns and Tunable Electronic and Optical Properties. J. Org. Chem. 2016, 81, 8394–8405. [Google Scholar] [CrossRef]
- Shi, J.; Fan, J.; Qu, Z.; Wang, S.; Wang, Y. Solution concentration-dependent tunable emission in cyclometalated iridium complex bearing perylene diimide (PDI) ligand: From visible to near-infrared emission. Dyes Pigm. 2018, 154, 263–268. [Google Scholar] [CrossRef]
- Schulze, M.; Steffen, A.; Wuerthner, F. Near-IR Phosphorescent Ruthenium(II) and Iridium(III) Perylene Bisimide Metal Complexes. Angew. Chem. Int. Ed. 2015, 54, 1570–1573. [Google Scholar] [CrossRef]

















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocard, L.; Goujon, A.; Hudhomme, P. Nitro-Perylenediimide: An Emerging Building Block for the Synthesis of Functional Organic Materials. Molecules 2020, 25, 1402. https://doi.org/10.3390/molecules25061402
Rocard L, Goujon A, Hudhomme P. Nitro-Perylenediimide: An Emerging Building Block for the Synthesis of Functional Organic Materials. Molecules. 2020; 25(6):1402. https://doi.org/10.3390/molecules25061402
Chicago/Turabian StyleRocard, Lou, Antoine Goujon, and Piétrick Hudhomme. 2020. "Nitro-Perylenediimide: An Emerging Building Block for the Synthesis of Functional Organic Materials" Molecules 25, no. 6: 1402. https://doi.org/10.3390/molecules25061402
APA StyleRocard, L., Goujon, A., & Hudhomme, P. (2020). Nitro-Perylenediimide: An Emerging Building Block for the Synthesis of Functional Organic Materials. Molecules, 25(6), 1402. https://doi.org/10.3390/molecules25061402