Ionotropic Gelation of Chitosan for Next-Generation Composite Proton Conducting Flat Structures
Abstract
:1. Introduction
1.1. Why Chitosan?
1.2. Why Phosphotungstic Acid?
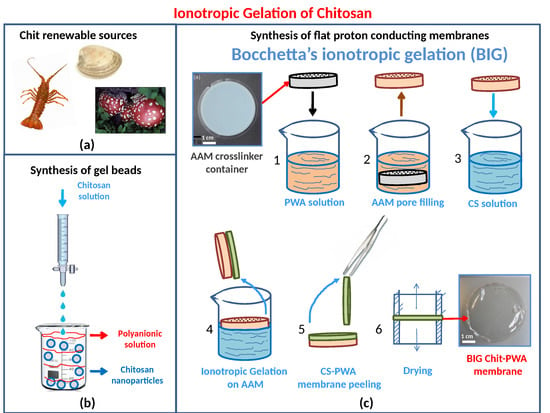
1.3. Methods for the Fabrication of Proton Conducting Chitosan-Based Membranes
2. Results and Discussion
2.1. In-Situ Ionotropic Gelation of Chitosan with Phosphotungstate
2.1.1. The BIG Method
2.1.2. Monitoring the BIG Reaction and the Prolonged Post-Crosslinking Treatments
3. Materials and Methods
3.1. Materials
3.2. The Bocchetta’s Ionotropic Gelation (BIG) Procedure
3.3. Characterization of the Chit/PWA Composite Membranes
3.4. Fuel Cell Assembling and Measurements
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Vårum, K.M.; Smidsrød, O. Structure-Property Relationship in Chitosans. In Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 625–642. [Google Scholar]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. Chem. Bio. Eng. Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Author, C.; Jafarizadeh Malmiri, H.; Ali Ghaz Jahanian, M.; Berenjian, A. Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. Am. J. Biochem. Biotechnol. 2012, 8, 203–219. [Google Scholar]
- Sacco, P.; Borgogna, M.; Travan, A.; Marsich, E.; Paoletti, S.; Asaro, F.; Grassi, M.; Donati, I. Polysaccharide-Based Networks from Homogeneous Chitosan-Tripolyphosphate Hydrogels: Synthesis and Characterization. Biomacromolecules 2014, 15, 3396–3405. [Google Scholar] [CrossRef]
- Sacco, P.; Paoletti, S.; Cok, M.; Asaro, F.; Abrami, M.; Grassi, M.; Donati, I. Insight into the ionotropic gelation of chitosan using tripolyphosphateand pyrophosphate as cross-linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Teruel-Juanes, R.; Arenga, F.; Salaberria, A.M.; Baschetti, M.G.; Labidi, J.; Badia, J.D.; Ribes-Greus, A. Crosslinked chitosan/poly(vinyl alcohol)-based polyelectrolytes for proton exchange membrane. React. Funct. Polym. 2019, 142, 213–222. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Peters, M.G. Chitin Handbook; Università di Ancona: European Chitin Society, Breman, Germany, 1997; pp. 437–438. [Google Scholar]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Sashiwa, H.; Aiba, S. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Wu, H.; Hou, W.; Wang, J.; Xiao, L.; Jiang, Z. Preparation and properties of hybrid direct methanol fuel cell membranes by embedding organophosphorylated titania submicrospheres into a chitosan polymer matrix. J. Power Sources 2010, 195, 4104–4113. [Google Scholar] [CrossRef]
- Yuan, S.; Tang, Q.; He, B.; Chen, H.; Li, Q.; Ma, C.; Jin, S.; Liu, Z. H3PO4 imbibed polyacrylamide-graft-chitosan frameworks for high-temperature proton exchange membranes. J. Power Sources 2014, 249, 277–284. [Google Scholar] [CrossRef]
- Wang, S.; Shi, L.; Zhang, S.; Wang, H.; Cheng, B.; Zhuang, X.; Li, Z. Proton-conducting amino acid-modified chitosan nanofibers for nanocomposite proton exchange membranes. Eur. Polym. J. 2019, 119, 327–334. [Google Scholar] [CrossRef]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. Int. J. Mol. Sci. 2020, 21, 632. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, O.; Ogino, I. Electrical conductivities of some hydrates of dodecamolybdophosphoric acid and dodecatungstophosphoric acid and their mixed crystals. Mater. Res. Bull. 1982, 17, 231–234. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Su, H.; Zeng, J.; Jiang, S.P.; Goddard, W.A.J. Insight into proton transfer in phosphotungstic acid functionalized mesoporous silica-based proton exchange membrane fuel cells. Am. Chem. Soc. 2014, 136, 4954–4964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Honma, I. Biomembranes for fuel cell electrolytes employing anhydrous proton-conducting uracil composites. Fuel Cells Bull. 2006, 5, 11–15. [Google Scholar] [CrossRef]
- Bocchetta, P.; Conciauro, F.; Di Quarto, F. Nanoscale membrane electrode assemblies based on porous anodic alumina for hydrogen–oxygen fuel cell. J. Solid State Electrochem. 2007, 11, 1253–1261. [Google Scholar] [CrossRef]
- Bocchetta, P.; Conciauro, F.; Santamaria, M.; Di Quarto, F. Fuel Cell Performances of Bio-Membranes Made of Chitosan-Polyelectrolyte Thin Films and Nanowires into Anodic Alumina Membranes. Proceedings of 220th ECS Meeting, Boston, MA, USA, 9–14 October 2011. [Google Scholar]
- Zhai, L.; Li, H. Polyoxometalate–Polymer Hybrid Materials as Proton Exchange Membranes for Fuel Cell Applications. Molecules 2019, 24, 3425. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Liu, C.; Lu, T.; Xing, W. Polyelectrolyte complexes of chitosan and phosphotungstic acid as proton-conducting membranes for direct methanol fuel cells. J. Power Sources 2007, 167, 94–99. [Google Scholar] [CrossRef]
- Cui, Z.; Xing, W.; Liu, C.; Liao, J.; Zhang, H. Chitosan/heteropolyacid composite membranes for direct methanol fuel cell. J. Power Sources 2009, 188, 24–29. [Google Scholar] [CrossRef]
- Santamaria, M.; Pecoraro, C.M.; Di Quarto, F.; Bocchetta, P. Chitosan phosphotungstic acid complex as membranes for low temperature H2-O2 fuel cell. J. Power Sources 2015, 276, 189–194. [Google Scholar] [CrossRef]
- Pecoraro, C.M.; Santamaria, M.; Bocchetta, P.; Di Quarto, F. Influence of synthesis conditions on the performance of chitosan-heteropolyacid complexes as membranes for low temperature H2-O2 fuel cell. Int. J. Hydrog. Energy 2015, 40, 14616–14626. [Google Scholar] [CrossRef]
- Odeh, A.O.; Osifo, P.; Noemagus, H. Chitosan: A Low Cost Material for the Production of Membrane for Use in PEMFC-A Review. Energy Sources Part A Recov. Util. Env. Eff. 2013, 35, 152–163. [Google Scholar] [CrossRef]
- Muhmed, S.A.; Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Emerging Chitosan and Cellulose Green Materials for Ion Exchange Membrane Fuel Cell: A Review. Energy Ecol. Environ. 2019. [Google Scholar] [CrossRef]
- Xiaoa, Y.; Xianga, Y.; Xiua, R.; Lu, S. Development of cesium phosphotungstate salt and chitosan composite membrane for direct methanol fuel cells. Carbohydr. Polym. 2013, 98, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, H.; Lu, S.; Xu, X.; Liang, D.; Xiang, Y. Novel methanol-blocking proton exchange membrane achieved via self-anchoring phosphotungstic acid into chitosan membrane with submicro-pores. J. Memb. Sci. 2016, 500, 203–210. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Khastgir, D. Fabrication and comprehensive investigation of physicochemical and electrochemical properties of chitosan-silica supported silicotungstic acid nanocomposite membranes for fuel cell applications. Energy 2018, 142, 313–330. [Google Scholar]
- De Moraes, M.A.; Cocenza, D.S.; da Cruz Vasconcellos, F.; Fraceto, L.F.; Beppu, M.M. Chitosan and alginate biopolymer membranes for remediation of contaminated water with herbicides. J. Environ. Manag. 2013, 131, 222–227. [Google Scholar] [CrossRef]
- Mat, N.; Liong, A. Chitosan-poly (vinyl alcohol) and calcium oxide composite membrane for direct methanol fuel cell applications. Eng. Lett. 2009, 116, 1017–1029. [Google Scholar]
- Campos, M.G.N.; Ferreira Grosso, C.R.; Cárdenas, G.; Inocentinni Mei, L.H. Effects of Neutralization Process on Preparation and Characterization of Chitosan Membranes for Wound Dressing. Macromol. Symp. 2005, 229, 253–257. [Google Scholar] [CrossRef]
- Kaiser, V.; Stropnik, C.; Musil, V.; Brumen, B. Morphology of solidified polysulfone structures obtained by wet phase separation. Eur. Polym. J. 2007, 43, 2515–2524. [Google Scholar] [CrossRef]
- Patil, J.S.; Kamalapur, M.V.; Marapur, S.C.; Kadam, D.V. Ionotropic gelation and polyelectrolyte complexation: The novel techniques to design hydrogel particulate sustained, modulated drug delivery system: A review. Dig. J. Nanomater. Biostructures 2010, 5, 241–248. [Google Scholar]
- Henao, E.; Delgado, E.; Contreras, H.; Quintana, G. Polyelectrolyte Complexation versus Ionotropic Gelation for Chitosan-Based Hydrogels with Carboxymethylcellulose, Carboxymethyl Starch, and Alginic Acid. Int. J. Chem. Eng. 2018. [Google Scholar] [CrossRef] [Green Version]
- Malmo, J.; Sandvig, A.; Vårum, K.M.; Strand, S.P. Nanoparticle mediated P-glycoprotein silencing for improved drug delivery across the blood-brain barrier: A siRNA-chitosan approach. PLoS ONE 2013, e54182. [Google Scholar] [CrossRef] [Green Version]
- Debnath, S.; Suresh Kumar, R.; Babu, M.N. Ionotropic Gelation–A Novel Method to Prepare Chitosan Nanoparticles. Res. J. Pharm. Tech. 2011, 4, 492–495. [Google Scholar]
- Hashad, R.A.; Ishak, R.A.H.; Fahmy, S.; Mansour, S.; Geneidi, A.S. Chitosan-tripolyphosphate nanoparticles: Optimization of formulation parameters for improving process yield at a novel pH using artificial neural networks. Int. J. Biol. Macromol. 2016, 86, 50–58. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Monovalent salt enhances colloidal stability during the formation of chitosan/tripolyphosphate microgels. Langmuir 2011, 27, 10392–10399. [Google Scholar] [CrossRef]
- Bocchetta, P.; Conciauro, F.; Santamaria, M.; Di Quarto, F. Cs0.86(NH4)1.14SO4Te(OH)6 in porous anodic alumina for micro fuel cell applications. Electrochim. Acta 2011, 56, 3845–3851. [Google Scholar] [CrossRef]
- Bocchetta, P.; Santamaria, M.; Di Quarto, F. One-step electrochemical synthesis and physico-chemical characterization of CdSe nanotubes. Electrochim. Acta 2013, 88, 340–346. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Taktak, N.E.M.; Awad, O.M.; Elfiki, S.A.; EleEla, N.E.A. Preparation and characterization of biopolymers chitosan/alginate/gelatin gel spheres crosslinked by glutaraldehyde. J. Macromol. Sci. B 2017, 56, 359–372. [Google Scholar] [CrossRef]
- Leanirith, Y.; Bunel, C.; Vairon, J.P. Reversible immobilization of drugs on a hydrogel matrix, 2. Diffusion of free chloramphenicol from poly (2-hydroxyethyl methacrylate) hydrogels. Die Makromol. Chem. 1990, 191, 1119–1129. [Google Scholar]
- Peppas, N.A.; Franson, N.M. The swelling interface number as a criterion for prediction of diffusional solute release mechanisms in swellable polymers. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 983–997. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Neodini Remedio, L.; Wesley Silva dos Santos, J.; Maciel, V.; Borges, V.; Pedroso Yoshida, C.M.; de Carvalho, R.A. Characterization of active chitosan films as a vehicle of potassium sorbate or nisin antimicrobial agents. Food Hydrocoll. 2019, 87, 830–838. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Lin, Z. Kinetics and thermodynamics of the water sorption of 2-hydroxyethyl methacrylate/styrene copolymer hydrogels. J. Appl. Polym. Sci. 2008, 109, 3018–3023. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical description of hydrogel swelling: A review. Iran Polym. J. 2010, 19, 375–398. [Google Scholar]
- Kim, S.J.; Lee, K.J.; Kim, S.I. Swelling behavior of polyelectrolyte complex hydrogels composed of chitosan and hyaluronic acid. J. Appl. Polym. Sci. 2004, 93, 1097–1101. [Google Scholar] [CrossRef]
- Kim, B.; La Flamme, K.; Peppas, N.A. Dynamic swelling behavior of pH-sensitive anionic hydrogels used for protein delivery. J. Appl. Polym. Sci. 2003, 89, 1606–1613. [Google Scholar] [CrossRef]
- Khare, A.R.; Peppas, N.A. Swelling/deswelling of anionic copolymer gels. Biomaterials 1995, 16, 559–567. [Google Scholar] [CrossRef]
- Belamie, E.; Domard, A.; GiraudGuille, M.M. Study of the solid state hydrolysis of chitosan in presence of HCl. J. Polym. Sci. Pol. Chem. 1997, 35, 3181–3191. [Google Scholar] [CrossRef]
- Samuels, R.J. Solid state characterization of the structure of chitosan films. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1081–1105. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Jiang, Q.; Xia, W. Physicochemical and structural characteristics of chitosan nanopowders prepared by ultrafine milling. Carbohyd. Polym. 2012, 87, 309–313. [Google Scholar] [CrossRef]
- Cervera, M.F.; Heinamaki, J.; Rasanen, M.; Maunu, S.L.; Karjalainen, M.; Acosta, O.M.N.; Iraizoz Colarte, A.; Yliruusi, J. Solid-state characterization of chitosans derived from lobster chitin. Carbohyd. Polym. 2004, 58, 401–408. [Google Scholar] [CrossRef]
- Du, J.; Bai, Y.; Chu, W.; Qiao, L. The structure and electric characters of proton conducting chitosan membranes with various ammonium salts as complexant. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 880–885. [Google Scholar] [CrossRef]
- Peniche-Covas, C.; Arguelles-Monal, W.; San Román, J. A kinetic study of the thermal degradation of chitosan and a mercaptan derivative of chitosan. J. Polym. Degrad. Stab. 1993, 39, 24–28. [Google Scholar] [CrossRef]
- Mukomaa, P.; Joosteb, B.R.; Voslooc, H.C.M. Synthesis and characterization of cross-linked chitosan membranes for application as alternative proton exchange membrane materials in fuel cells. J. Power Sources 2004, 136, 16–23. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Thom, D. Characterisation of alginate composition and block-structure by circular dichroism. Carbohydr. Res. 1980, 81, 305–314. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207. [Google Scholar] [CrossRef]
- Cui, Z.; Xiang, Y.; Si, J.; Yang, M.; Zhang, Q.T. Zhang. Ionic interactions between sulfuric acid and chitosan membranes. Carbohydr. Polym. 2008, 73, 111–116. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yi, Z.; Wang, J.; Hou, T.; Jiang, Q. Carboxymethyl konjac glucomannan-crosslinked chitosan sponges for wound dressing. Int. J. Biol. Macromol. 2018, 112, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tain, R.; Rhodes, C. A study of the decomposition behaviour of 12-tungstophosphate heteropolyacid in solution. Can. J. Chem. 2003, 81, 1044–1050. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Chit-PWA proton conducting membranes are available from the author. |








© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocchetta, P. Ionotropic Gelation of Chitosan for Next-Generation Composite Proton Conducting Flat Structures. Molecules 2020, 25, 1632. https://doi.org/10.3390/molecules25071632
Bocchetta P. Ionotropic Gelation of Chitosan for Next-Generation Composite Proton Conducting Flat Structures. Molecules. 2020; 25(7):1632. https://doi.org/10.3390/molecules25071632
Chicago/Turabian StyleBocchetta, Patrizia. 2020. "Ionotropic Gelation of Chitosan for Next-Generation Composite Proton Conducting Flat Structures" Molecules 25, no. 7: 1632. https://doi.org/10.3390/molecules25071632
APA StyleBocchetta, P. (2020). Ionotropic Gelation of Chitosan for Next-Generation Composite Proton Conducting Flat Structures. Molecules, 25(7), 1632. https://doi.org/10.3390/molecules25071632