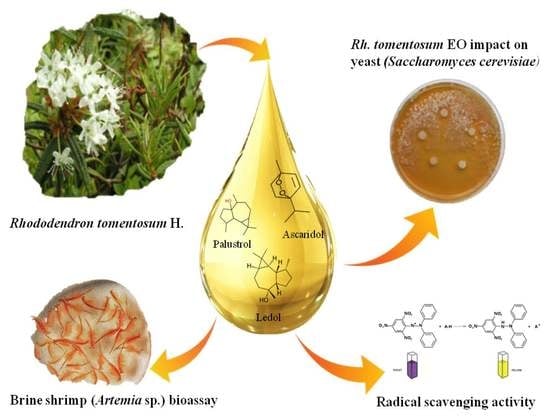
Toxic, Radical Scavenging, and Antifungal Activity of Rhododendron tomentosum H. Essential Oils
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Essential Oils
2.2. Toxic Activity
2.3. Radical Scavenging Activity
2.4. Antifungal Activity
2.4.1. Antifungal Activity of EO against Yeast Tested by Agar Disc Diffusion Method
2.4.2. Effect of Rh. Tomentosum EO Volatile Compounds on Yeast S. cerevisiae Membrane Permeability
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Preparation
4.3. GC (Flame-Ionization Detector FID) Analysis
4.4. GC-MS Analysis
4.5. Identification of Individual Components
4.6. Toxicity Test
4.7. Antioxidant Activity
4.7.1. ABTS•+ Assay
4.7.2. DPPH• Assay
4.7.3. TROLOX Equivalent Assay
4.8. Antifungal Activity
4.8.1. Agar Disc Diffusion Method for Testing Antifungal Activity of EO against Yeast
4.8.2. Amperometric Study of Rh. tomentosum Essential Oil Effect on S. cerevisiae Membrane Permeability
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Dampc, A.; Luczkiewicz, M. Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinric, M. Review: Medicinal plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sõukand, R.; Kalle, R.; Svanberg, I. Uninvited guests: Traditional insect repellents in Estonia used against the clothes moth Tineola bisselliella, human flea Pulex irritans and bedbug Cimex lectularius. J. Insect Sci. 2010, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Ylipahkala, J.E.; Jalonen, T.M. Isolation of very volatile compounds from the leaves of Ledum palustre using the purge & trap technique. Chromatography 1992, 34, 4159–4162. [Google Scholar]
- Holm, Y.; Laakso, I.; Harmaja, H.; Hiltunen, R. The essential oils of Ledum palustre L.: An ascaridol chemotype. In Proceedings of the 24th International Symposium on Essential Oils, Final Programme, Abstracts, List of Participants, Berlin, Germany, 21–24 July 1993; p. 50. [Google Scholar]
- Sozinov, O.V.; Kuzmicheva, N.A. Cenopopulations of Ledum palustre L. and their plant material characteristics under conditions of Srednenemanskaja lowland (Republic of Belarus). Rast. Resursy 2003, 39, 55–62. (In Russian) [Google Scholar]
- Shavarda, A.L.; Khanin, V.A.; Medvedeva, N.A.; Danchul, T.Y.; Shagova, L.V. Characteristics of volatile terpenoid content in shoots of Ledum palustre L. (Leningrad region). Rast. Resursy 2004, 40, 87–95. (In Russian) [Google Scholar]
- Jaenson, T.G.T.; Pålsson, K.; Borg-Karlson, A.-K. Evaluation of extracts and oils of tick-repellent plants from Sweden. Med. Vet. Entomol. 2005, 19, 345–352. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Pålsson, K.; Borg-Karlson, A.-K. Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. J. Med. Entomol. 2006, 43, 113–119. [Google Scholar] [CrossRef]
- Butkienė, R.; Šakočiūtė, V.; Latvėnaitė, D.; Mockutė, D. Composition of young and aged shoot essential oils of the wild Ledum palustre L. Chemija 2008, 19, 19–24. [Google Scholar]
- Gretšušnikova, T.; Järvan, K.; Orav, A.; Koel, M. Comparative analysis of the composition of the essential oil from the shoots, leaves and stems of the wild Ledum palustre L. from Estonia. Proced. Chem. 2010, 2, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Butkiene, R.; Mockute, D. The variability of the essential oil composition of wild Ledum palustre L. shoots during vegetation period. J. Essent. Oil Res. 2011, 23, 9–13. [Google Scholar] [CrossRef]
- Judžentienė, A.; Būdienė, J.; Misiūnas, A.; Butkienė, R. Variation in essential oil composition of Rhododendron tomentosum gathered in limited population (in Eastern Lithuania). Chemija 2012, 23, 131–135. [Google Scholar]
- Judzentiene, A.; Butkiene, R.; Budiene, J.; Tomi, F.; Casanova, J. Composition of seed essential oils of Rhododendron tomentosum. Nat. Prod. Commun. 2012, 7, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Raal, A.; Orav, A.; Gretchushnikova, T. Composition of the essential oil of the Rhododendron tomentosum Harmaja from Estonia. Nat. Prod. Res. 2014, 28, 1091–1098. [Google Scholar] [CrossRef]
- Jesionek, A.; Poblocka-Olech, L.; Zabiegala, B.; Bucinski, A.; Krauze-Baranowska, M.; Luczkiewicz, M. Validated HPTLC method for determination of ledol and alloaromadendrene in the essential oil fractions of Rhododendron tomentosum plants and in vitro cultures and bioautography for their activity screening. J. Chromatogr. B Biomed. Sci. Appl. 2018, 1086, 63–72. [Google Scholar] [CrossRef]
- Jesionek, A.; Zabiegala, B.; Bucinski, A.; Luczkiewicz, M. From harvesting to distillation–Effect of analytical procedures on the yield and chemical composition of Rhododendron tomentosum (Ledum palustre) essential oil. Acta Pol. Pharm. 2019, 76, 83–92. [Google Scholar]
- Jesionek, A.; Kokotkiewicz, A.; Mikosik-Roczynska, A.; Ciesielska-Figlon, K.; Luczkiewicz, P.; Bucinski, A.; Daca, A.; Witkowski, J.M.; Bryl, E.; Zabiegala, B.; et al. Chemical variability of Rhododendron tomentosum (Ledum palustre) essential oils and their pro-apoptotic effect on lymphocytes and rheumatoid arthritis synoviocytes. Fitoterapia 2019, 139, 104402. [Google Scholar] [CrossRef]
- Belousova, N.I.; Khan, V.A. Bicyclic monoterpenoids of the essential oil of Ledum palustre. Khim. Prirod. Soed. 1990, 5, 627–629. [Google Scholar] [CrossRef]
- Belousova, N.I.; Khan, V.A.; Berezovskaya, T.P. Intraspecies chemical variability of the essential oil of Ledum palustre. Khim. Prirod. Soed. 1990, 4, 472–480. [Google Scholar] [CrossRef]
- Belousova, N.I.; Khan, V.A.; Berezovskaya, T.P.; Salenko, V.L.; Velkov, A.I.; Dmitruk, S.E. Composition of essential oils of Ledum palustre L. from Tomsk region. Rast. Resursy 1991, 3, 81–89. (In Russian) [Google Scholar]
- Belousova, N.I.; Tkachev, A.V.; Shakirov, M.M.; Khan, V.A. New terpenoids of the essential oil of Ledum palustre. Khim. Prirod. Soed. 1991, 1, 24–29. [Google Scholar] [CrossRef]
- Petrov, K.A. The effect of essential oils of Ledum palustre L., Acorus calamus L., Artemisia jacutica Drob. on the biotests growth. Rast. Resursy 1998, 34, 47–50. (In Russian) [Google Scholar]
- Tkachev, A.V.; Belousova, N.I.; Khan, V.A. 7-Elemol—New sesquiterpene alcohol from ethereal oil of Ledum palustre L. Chem. Plant Raw. Mater. 1999, 3, 39–40. [Google Scholar]
- Zhao, D.-x.; Wang, H.-t.; Wu, C.-s.; Sun, S.-w.; Ma, Y.-p. The main chemical components of essential oil from Ledum palustre L. var. angustum N. Busch. Acta Bot. Sinica 1987, 29, 189–192. [Google Scholar]
- Šatar, S. Untersuschungen der chemischen Zusammensetzung des atherischenoles von Ledum palustre aus der Mongolei. Pharmazie 1988, 43, 293. [Google Scholar]
- Ueyama, Y.; Hashimoto, S.; Nii, H.; Furukawa, K. Constituents of the essential oil from Ledum palustre L. var. angustum N. Busch. Nippon Nogeik Kagakukaishi 1989, 63, 849–851. [Google Scholar] [CrossRef]
- Zhi Guo, Q.; Cheng Zeng, Y.; Xue Fei, G.; Hui Yuan, M. Separation and identification of main components of Ledum oil. J. Northeast Forest. Univ. 1997, 25, 41–44. [Google Scholar]
- Zhao, Z.; Wang, Y.; Du, X.; Liu, X.; Li, D.; Sun, Z. Study on the composition and application of essential oil of Ledum palustre L. var angustum. Chem. Industr. Forest Prod. 2001, 35, 3–5. [Google Scholar]
- Xiu, Z.L.; Zhu, X.L.; Zhang, D.J.; Yin, J.Z.; Wang, D.H.; An, L.J. A new way for chemical degradation of plastic by natural volatile constituents of Ledum palustre. Chin. Sci. Bull. 2003, 48, 1718–1721. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M. Study on the volatile constituents of Ledum palustre L. in the Daxingan Mountains. Chin. J. Chromatogr. 2003, 21, 631. [Google Scholar]
- Kim, D.; Nam, B. Extracts and essential oil of Ledum palustre L. and their antioxidant and antimicrobial activities. J. Food Sci. Nutr. 2006, 11, 100–104. [Google Scholar] [CrossRef]
- Chen, Q.X.; Wang, X.Q.; Chen, Q.H.; Wen, C.H. The study of extraction methods of the volatile oil of Ledum palustre. Acta Acad. Med. Neimongol. 2006, 28, 414–416. [Google Scholar]
- Qu, G.; Li, B.F.; Zhao, H.M.; Du, F.G.; Sun, G.S.; Wang, R.J. Chemical composition and application as biomaterials of essential oils from Ledum palustre of Changbai Mountain China. Appl. Mech. Mater. 2014, 568, 1907–1910. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, Q.; Yuan, G.; Xu, F.; Li, B.; Wang, J.; Ouyang, J. Comparison of the essential oil composition of wild Rhododendron tomentosum stems, leaves, and flowers in bloom and non-bloom periods from Northeast China. J. Essent. Oil Bear. Pl. 2016, 19, 1216–1223. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, H.X.; Wang, Y.M.; Xu, M.; Hu, X.Y. Diurnal effects on Chinese wild Ledum palustre L. essential oil yields and composition. J. Anal. Sci. Methods Instrum. 2017, 7, 47–55. [Google Scholar]
- Kuusik, A.; Harak, M.; Hiiesaar, K.; Metspalu, L.; Tartes, U. Studies on insect growth regulating (IGR) and toxic effects of Ledum palustre extracts on Tenebrio molitor pupae (Coleoptera, Tenebrionidae) using calorimetric recordings. Termochim. Acta 1995, 251, 247–253. [Google Scholar] [CrossRef]
- Egigu, M.C.; Ibrahim, M.A.; Yahya, A.; Holopainen, J.K. Cordeauxia edulis and Rhododendron tomentosum extracts disturb orientation and feeding behavior of Hylobius abietis and Phyllodecta laticollis. Entomol. Exp. Appl. 2011, 138, 162–174. [Google Scholar] [CrossRef]
- Himanen, S.J.; Bui, T.N.T.; Mengistu, M.M.; Holopainen, J.K. Utilizing associational resistance for biocontrol: Impacted by temperature, supported by indirect defense. BMC Ecol. 2015, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhao, J.-j.; Wu, W.-b.; Zhao, Y.; Zhu, X.-l.; Qu, W.-j. Analgesic and anti-inflammatory activities of extracts from Ledum palustre L. in mice. Nat. Prod. Res. Dev. 2010, 22, 326–329. [Google Scholar]
- Belousov, M.V.; Saratikov, A.S.; Akhmedzhanov, R.R.; Berezovskaya, T.A.; Yusubov, M.S.; Dmitruk, S.E.; Basova, E.V. Pharmacological properties of Ledum palustre (Ericaceae) from Tomsk region. Rast. Resursy 2006, 42, 130–140. (In Russian) [Google Scholar]
- Goun, E.A.; Petrichenko, V.M.; Solodnikov, S.U.; Suhinina, T.V.; Kline, M.A.; Cunningham, G.; Nguyen, C.; Miles, H. Anticancer and antithrombin activity of Russian plants. J. Ethnopharm. 2002, 81, 337–342. [Google Scholar] [CrossRef]
- Spiridonov, N.A.; Konovalov, D.A.; Arkhipov, V.V. Cytotoxicity of some Russian ethnomedicinal plants and plant compounds. Phytother. Res. 2005, 19, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Harbilas, D.; Martineau, L.C.; Harris, C.S.; Adeyiwola-Spoor, D.C.; Saleem, A.; Lambert, J.; Caves, D.; Johns, T.; Prentki, M.; Cuerrier, A.; et al. Evaluation of the antidiabetic potential of selected medicinal plant extracts from the Canadian boreal forest used to treat symptoms of diabetes: Part II. Can. J. Physiol. Pharm. 2009, 8, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Nistor Baldea, L.A.; Martineau, L.C.; Benhaddou-Andaloussi, A.; Arnason, J.T.; Lévy, É.; Haddad, P.S. Inhibition of intestinal glucose absorption by anti-diabetic medicinal plants derived from the James Bay Cree traditional pharmacopeia. J. Ethnopharmacol. 2010, 132, 473–482. [Google Scholar] [CrossRef]
- Belousova, N.I.; Dmitruk, S.E.; Khan, V.A. Antifungal properties of essential oils of Ledum L. Khim. Farm. Zh. 1989, 23, 317–319. [Google Scholar]
- Jin, C.; Strembiski, W.; Kulchytska, Y.; Micetich, R.G.; Daneshtalab, M. Flavonoid glycosides from Ledum palustre subsp. decumbens. Daru 1999, 7, 5–8. [Google Scholar]
- Baananou, S.; Bagdonaite, E.; Marongiu, B.; Piras, A.; Porcedda, S.; Falconieri, D.; Boughattas, N.A. Supercritical CO2 extract and essential oil of aerial part of Ledum palustre L.–Chemical composition and anti-inflammatory activity. Nat. Prod. Res. 2015, 29, 999–1005. [Google Scholar] [CrossRef]
- Wagner, H.; Wierer, M.; Bauer, R. In vitro Hemmung der Prostaglandin-Biosynthese durch etherische Ole und phenolische Verbindungen. Planta Med. 1986, 3, 184–187. [Google Scholar] [CrossRef]
- Tunón, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef]
- Zhang, L. Chemical composition and antibacterial activity of the essential oil from Chinese wild Ledum palustre L. on Vibrio Parahaemolyticus. Int. J. Food Nutr. Sci. 2017, 4, 8–12. [Google Scholar] [CrossRef]
- Belousov, M.V.; Saratikov, A.S.; Tshichalin, V.S.; Akhmedganov, R.R.; Berezovskaya, T.P.; Basova, E.V.; Yusubov, M.S. Hepatoprotective properties of Ledum palustre (Ericaceae) shoots dry extract. Rast. Resursy 2007, 43, 126–129. [Google Scholar]
- Basova, E.V. Chemical and Pharmacological Study of Wild Rosemary. Ph.D. Thesis, Tomsk State University, Tomsk, Russia, 2004. [Google Scholar]
- Narimanov, A.A. Reproductive capacity of male mice protected from supralethal effect of gamma-radiation by the administration of a mixture of extracts from Archangelica officinalis and Ledum palustre. Radiobiologia 1992, 32, 271–275. [Google Scholar]
- Narimanov, A.A.; Myakisheva, S.N.; Kuznetsova, S.M. The radioprotective effect of Archangelica officinalis Hoffm. and Ledum palustre L. extracts on mice. Radiobiologia 1991, 31, 391–393. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Quadrupole Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Puplishing Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Balouri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gijsen, H.; Wijnberg, J.; de Groot, A. Structure, occurrence, biosynthesis, biological activity, synthesis and chemistry of aromadendrane sesquiterpenoids. Fortschr. Chem. Org. Naturst. 1995, 64, 149–193. [Google Scholar]
- Tran, D.N.; Cramer, N. Biomimetic synthesis of (+)-ledene, (+)-viridiflorol, (-)-palustrol, (+)-spathulenol, and psiguadial A, C, and D via the platform terpene (+)-bicyclogermacrene. Chem. Eur. J. 2014, 20, 10654–10660. [Google Scholar] [CrossRef]
- Dembitsky, V.; Shkrob, I.; Hanus, L.O. Ascaridole and related peroxides from the genus Chenopodium. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2008, 152, 209–215. [Google Scholar] [CrossRef]
- Al-Footy, K.O.; Alarif, W.M.; Asiri, F.; Aly, M.M.; Ayyad, S.E.N. Rare pyrane-based cembranoids from the Red Sea soft coral Sarcophyton troheliophorum as potential antimicrobial-antitumor agents. Med. Chem. Res. Khim. Farm. Zh. 2014, 24, 505–512. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef]
- Mathew, J.; Thoppil, J.E. Chemical composition and mosquito larvicidal activities of Salvia essential oils. Pharm. Biol. 2011, 49, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, M.J.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácsera, A. Candida parapsilosis: From genes to the bedside. Clin. Microbiol. Rev. 2019, 32, e00111–e00118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, A.; Kavanagh, K. Emergence of Saccharomyces cerevisiae as a human pathogen. Implications for biotechnology. Enz. Microbiol. Technol. 1999, 25, 551–557. [Google Scholar] [CrossRef]
- Perez-Torrado, R.; Querol, A. Opportunistic strains of Saccharomyces cerevisiae: A potential risk sold in food products. Front. Microbiol. 2016, 6, 1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Rest, M.E.; Kammings, A.H.; Nakano, A.; Anraku, Y.; Poolman, B.; Konnings, W.N. The plasma membrane of Saccharomyces cerevisiae: Structure, function, and biogenesis. Microbiol. Rev. 1995, 59, 304–322. [Google Scholar] [CrossRef]
- D’Souza, S.F. Microbial biosensors. Biosens. Bioelectron. 2001, 16, 337–353. [Google Scholar] [CrossRef]
- Chen, R.R. Permeability issues in whole-cell bioprocesses and cellular membrane bioengineering. Appl. Microbiol. Biotechnol. 2007, 74, 730–738. [Google Scholar] [CrossRef]
- Da Cruz Almeida, E.T.; de Souza, G.; de Sousa Guedes, J.P.; Barbosa, I.M.; de Sousa, C.P.; Castellano, L.R.C.; Magnani, M.; de Souza, E.L. Mentha piperita L. essential oil inactivates spoilage yeasts in fruit juices through the perturbation of different physiological functions in yeast cells. Food Microbiol. 2019, 82, 20–29. [Google Scholar] [CrossRef]
- Garjonyte, R.; Melvydas, V.; Malinauskas, A. Effect of yeast pretreatment on the characteristics of yeast-modified electrodes as mediated amperometric biosensors for lactic acid. Bioelectrochemistry 2008, 74, 188–194. [Google Scholar] [CrossRef]
- McLaughlin, J.L.; Rogers, L.L.; Anderson, J.E. The use of biological assays to evaluate botanicals. Drug Inform. J. 1998, 32, 513–524. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




| Compounds | RI DB-5 # Exp/Lit | RI HP-FFAP ## Exp/Lit | 1 Sh April | SD (n = 5) | 2 Sh May | SD (n = 4) | 3 Fl May | SD (n = 3) | 4 Sh June | SD (n = 4) | 5 Sh July | SD (n = 5) | 6 Sh August | SD (n = 4) | 7 Sh September | SD (n = 5) | 8 Sh October | SD (n = 4) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myrcene | 991/990 | 1160/1160 | 1.8 ± 0.2 | 0.15 | 0.8 ± 0.1 | 0.05 | 10.1 ± 1.3 | 1.30 | 7.2 ± 0.3 | 0.25 | 1.8 ± 0.2 | 0.18 | 0.4 ± 0.1 | 0.10 | 1.8 ± 0.1 | 0.09 | 0.5 ± 0.1 | 0.08 |
| p-Cymene | 1025/1024 | 1270/1272 | 2.7 ± 0.2 | 0.28 | 2.8 ± 0.1 | 0.06 | 1.5 ± 0.2 | 0.28 | 1.8 ± 0.4 | 0.35 | 4.0 ± 0.4 | 0.29 | 4.8 ± 0.2 | 0.16 | 2.1 ± 0.1 | 0.08 | 0.2 ± 0.2 | 0.17 |
| 2-Methyl-6-methylene-3,7-octadien-2-ol | 1095/1095 | 1630/1630 | 1.3 ± 0.4 | 0.49 | 0.5 ± 0.2 | 0.17 | 2.2 ± 0.2 | 0.21 | 2.6 ± 0.3 | 0.25 | 1.5 ± 0.2 | 0.15 | 2.8 ± 0.4 | 0.33 | 3.5 ± 0.6 | 0.45 | 2.6 ± 0.2 | 0.22 |
| 2-Methyl-6-methylene-1,7-octadien-3-ol | 1158/1152 | 1700? | 1.7 ± 0.3 | 0.24 | 1.5 ± 0.1 | 0.08 | 1.3 ± 0.1 | 0.10 | 0.1 ± 0.1 | 0.08 | 0.1 ± 0.1 | 0.08 | 1.8 ± 0.2 | 0.17 | 4.5 ± 0.4 | 0.30 | 1.7 ± 0.4 | 0.33 |
| Lepalone a | 1258/1256 | 1757/1755 | 1.9 ± 0.2 | 0.22 | 2.7 ± 0.2 | 0.17 | 1.6 ± 0.4 | 0.40 | 2.3 ± 0.2 | 0.18 | 1.4 ± 0.2 | 0.17 | 2.4 ± 0.3 | 0.27 | 3.2 ± 0.2 | 0.16 | 3.5 ± 0.1 | 0.08 |
| Lepalol b | 1282/1281 | 2035/2034 | 3.3 ± 0.3 | 0.26 | 2.3 ± 0.5 | 0.42 | 1.6 ± 1.1 | 1.10 | 0.7 ± 0.5 | 0.43 | 0.1 ± 0.1 | 0.09 | 0.1 ± 0.1 | 0.10 | 3.2 ± 0.6 | 0.46 | 7.9 ± 0.3 | 0.28 |
| Bornyl acetate | 1290/1288 | 1578/1576 | 0.6 ± 0.1 | 0.10 | 0.7 ± 0.1 | 0.18 | 0.9 ± 0.1 | 0.12 | 3.3 ± 0.6 | 0.51 | 1.5 ± 0.6 | 0.55 | 2.8 ± 0.6 | 0.49 | 0.3 ± 0.1 | 0.08 | - | |
| iso-Ascaridol * | 1304/1307 | 1836/1836 | 3.0 ± 0.5 | 0.38 | 3.0 ± 0.2 | 0.16 | 1.4 ± 0.6 | 0.60 | 5.3 ± 1.6 | 1.31 | 14.0 ± 2.4 | 1.83 | 12.7 ± 2.0 | 1.65 | 7.0 ± 2.4 | 1.97 | 0.1 ± 0.1 | 0.10 |
| Palustrol | 1569/1568 | 1920/1934 | 33.5 ± 4.4 | 3.48 | 31.3 ± 1.3 | 1.06 | 30.0 ± 1.6 | 1.60 | 24.6 ± 2.6 | 2.13 | 25.2 ± 1.7 | 1.28 | 26.2 ± 2.0 | 1.66 | 26.0 ± 2.5 | 1.89 | 30.4 ± 5.0 | 4.30 |
| Ledol | 1604/1602 | 2026/2043 | 29.0 ± 5.0 | 4.06 | 27.5 ± 2.0 | 1.64 | 23.3 ± 2.3 | 2.30 | 18.0 ± 2.9 | 2.47 | 21.1 ± 2.5 | 2.06 | 21.6 ± 3.0 | 2.45 | 21.5 ± 4.0 | 2.86 | 28.0 ± 2.1 | 1.90 |
| Cyclocolorenone + epi-Cyclocolorenone | 1770/1760 | 2326/2326 | 3.2 ± 2.0 | 1.61 | 4.1 ± 2.5 | 2.04 | 4.1 ± 1.6 | 1.33 | 4.0 ± 0.5 | 0.43 | 2.7 ± 0.5 | 0.36 | 3.0 ± 0.1 | 0.08 | 4.2 ± 0.5 | 0.40 | 6.2 ± 0.3 | 0.25 |
| Total | 95.2 ± 4.1 | 3.61 | 96.2 ± 2.6 | 0.35 | 91.0 ± 4.7 | 4.70 | 95.0 ± 2.0 | 1.66 | 95.2 ± 3.0 | 2.01 | 93.7 ± 4.3 | 3.69 | 96.2 ± 3.1 | 2.29 | 96.1 ± 2.3 | 1.95 | ||
| Oxygenated sesquiterpenes | 76.1 ± 4.5 | 3.18 | 67.7 ± 2.0 | 1.27 | 64.8 ± 2.3 | 2.30 | 56.8 ± 4.0 | 3.57 | 54.1 ± 1.5 | 1.11 | 59.6 ± 2.2 | 1.83 | 60.0 ± 3.0 | 2.12 | 75.9 ± 2.2 | 1.80 |
| Artemia sp. nauplii Lethality | 1 Sh April (SD) | 2 Sh May (SD) | 3 Fl May (SD) | 4 Sh June (SD) | 5 Sh July (SD) | 6 Sh August (SD) | 7 Sh September (SD) | 8 Sh October (SD) |
|---|---|---|---|---|---|---|---|---|
| LC50, µg/mL | 14.06 (0.10) | 16.10 (2.10) | 20.50 (1.71) | 13.59 (2.71) | 14.43 (1.15) | 15.97 (1.55) | 11.23 (1.47) | 11.73 (0.73) |
| LC95, µg/mL | 55.95 (3.70) | 46.94 (7.52) | 76.07 (3.86) | 34.66 (2.84) | 41.58 (3.83) | 43.90 (5.30) | 34.00 (1.37) | 34.83 (1.86) |
| EO | 4 Sh June (SD) | 5 Sh July (SD) | 7 Sh September (SD) | 8 Sh October (SD) |
|---|---|---|---|---|
| TROLOX (mmol/L) | 48.19 ± 0.1 (0.01) | 31.41 ± 0.2 (0.02) | 30.35 ± 0.03 (0.03) | 16.25 ± 0.2 (0.28) |
| EO | 2 Sh May (SD) | 3 Fl May (SD) | 4 Sh June (SD) | 5 Sh July (SD) | 6 Sh August (SD) | 7 Sh September (SD) | 8 Sh October (SD) |
|---|---|---|---|---|---|---|---|
| TROLOX (mmol/L) | 11.92 ± 0.5 (0.5) | 5.86 ± 0.07 (0.07) | 19.89 ± 0.3 (0.03) | 17.82 ± 0.5 (0.05) | 12.07 ± 0.7 (0.7) | 14.97 ± 0.5 (0.5) | 12.26 ± 0.5 (0.5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judzentiene, A.; Budiene, J.; Svediene, J.; Garjonyte, R. Toxic, Radical Scavenging, and Antifungal Activity of Rhododendron tomentosum H. Essential Oils. Molecules 2020, 25, 1676. https://doi.org/10.3390/molecules25071676
Judzentiene A, Budiene J, Svediene J, Garjonyte R. Toxic, Radical Scavenging, and Antifungal Activity of Rhododendron tomentosum H. Essential Oils. Molecules. 2020; 25(7):1676. https://doi.org/10.3390/molecules25071676
Chicago/Turabian StyleJudzentiene, Asta, Jurga Budiene, Jurgita Svediene, and Rasa Garjonyte. 2020. "Toxic, Radical Scavenging, and Antifungal Activity of Rhododendron tomentosum H. Essential Oils" Molecules 25, no. 7: 1676. https://doi.org/10.3390/molecules25071676
APA StyleJudzentiene, A., Budiene, J., Svediene, J., & Garjonyte, R. (2020). Toxic, Radical Scavenging, and Antifungal Activity of Rhododendron tomentosum H. Essential Oils. Molecules, 25(7), 1676. https://doi.org/10.3390/molecules25071676