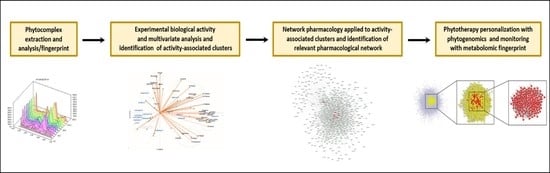
Essential Oil Phytocomplex Activity, a Review with a Focus on Multivariate Analysis for a Network Pharmacology-Informed Phytogenomic Approach
Abstract
:1. From Traditional Use of Essential Oils to Phytocomplex Molecular Characterization
2. Identification and Isolation of Bioactive Compounds and Derivatives from Essential Oils
3. Oneness and Multiplicity of the Phytocomplex: Pushing Too Far the Reductionist Approach Can Lead to Biological Irrelevance
4. Phytochemical Research, the Emergence of the Holistic Approach
5. Network Pharmacology Meets the Phytocomplex
6. Analytical Strategies Fit for Studying Phytocomplexes
7. Multivariate Approach to Study Essential Oils’ Biological Activity
8. Network Pharmacology Guided Phytogenomics for Personalized Medicine
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kubeczka, K.-H. History and Sources of Essential Oil Research. In Handbook of Essential Oils; Informa UK Limited: London, UK, 2009; pp. 3–38. [Google Scholar]
- Guenther, E. The Essential Oils-Vol 1: History-Origin in Plants-Production-Analysis; Read Books Limited: Redditch, UK, 2013. [Google Scholar]
- Franz, C.; Novak, J. Sources of Essential Oils. In Handbook of Essential Oils; Informa UK Limited: London, UK, 2009; pp. 39–81. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Guenther, E. The essential oils; D. Van Nostrand Company, Inc.: New York, NY, USA, 1948. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Cohen, S.; Doull, J.; Feron, V.; Goodman, J.; Marnett, L.; Portoghese, P.; Waddell, W.; Wagner, B.; Hall, R.; et al. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical Composition, Seasonal Variability, and Antifungal Activity ofLavandula stoechasL. ssp.stoechasEssential Oils from Stem/Leaves and Flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef] [PubMed]
- Fathollahi, M.; Aminzare, M.; Mohseni, M.; Hassanzadazar, H. Antioxidant capacity, antimicrobial activities and chemical composition of Pistacia atlantica subsp. kurdica essential oil. Vet. Res. Forum 2019, 10, 299–305. [Google Scholar] [PubMed]
- Donato, R.; Sacco, C.; Pini, G.; Bilia, A.R.; Rosa, D.; Cristiana, S.; Gabriella, P.; Bilia, A.R. Antifungal activity of different essential oils against Malassezia pathogenic species. J. Ethnopharmacol. 2020, 249, 112376. [Google Scholar] [CrossRef]
- Van Vuuren, S.; De Rapper, S. Odoriferous Therapy: Identifying the Antimicrobial Potential of Essential Oils against Pathogens of the Respiratory Tract. Chem. Biodivers. 2020. [Google Scholar] [CrossRef]
- Danzi, D.; Ladu, G.; Prieto, C.V.; Bullon, A.G.; Petretto, G.L.; Fancello, F.; Venditti, T. Antifungal activity in vitro and on food of the essential oil extracted from pompia (Citrus limon var. pompia) leaves against Penicillium digitatum applied by vapor contact. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- Fitsiou, E.; Pappa, A. Anticancer Activity of Essential Oils and Other Extracts from Aromatic Plants Grown in Greece. Antioxidants 2019, 8, 290. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, P.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Dias, H.J.; Crotti, A.E.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farm. 2015, 25, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharbach, M.; Marmouzi, I.; El Jemli, M.; Bouklouze, A.; Heyden, Y.V. Recent advances in untargeted and targeted approaches applied in herbal-extracts and essential-oils fingerprinting—A review. J. Pharm. Biomed. Anal. 2019, 177, 112849. [Google Scholar] [CrossRef] [PubMed]
- Edris, A. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.K.; Sachan, N.K.; Kumar, S.; Sachan, A.; Gangwar, S.S. Evaluation and standardization of essential oils for development of alternative dosage forms. Eur. J. Sci. Res. 2010, 46, 194–203. [Google Scholar]
- Agarwal, A.; D’Souza, P.; Johnson, T.S.; Dethe, S.; Chandrasekaran, C. Use of in vitro bioassays for assessing botanicals. Curr. Opin. Biotechnol. 2014, 25, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.G. A Unifying Review of Bioassay-Guided Fractionation, Effect-Directed Analysis and Related Techniques. Sensors 2012, 12, 9181–9209. [Google Scholar] [CrossRef]
- He, J. Bioactivity-Guided Fractionation of Pine Needle Reveals Catechin as an Anti-hypertension Agent via Inhibiting Angiotensin-Converting Enzyme. Sci. Rep. 2017, 7, 8867. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Elson, C.E.; Maltzman, T.H.; Boston, J.L.; Tanner, M.A.; Gould, M.N. Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis. Carcinogenesis 1988, 9, 331–332. [Google Scholar] [CrossRef]
- Juteau, F.; Masotti, V.; Bessière, J.M.; Dherbomez, M.; Viano, J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia 2002, 73, 532–535. [Google Scholar] [CrossRef]
- Didry, N.; Dubreuil, L.; Pinkas, M. Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm. Acta Helv. 1994, 69, 25–28. [Google Scholar] [CrossRef]
- Cortelli, S.C.; Cortelli, J.R.; Holzhausen, M.; Franco, G.C.N.; Rebelo, R.Z.; Sonagere, A.S.; Queiroz, C.; Costa, F.O. Essential oils in one-stage full-mouth disinfection: Double-blind, randomized clinical trial of long-term clinical, microbial and salivary effects. J. Clin. Periodontol. 2009, 36, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Aghajanshakeri, S.; Mikaili, P.; Mojaverrostami, S. Pharmacological and therapeutic effects of Mentha Longifolia, L. and its main constituent, menthol. Anc. Sci. Life 2013, 33, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, S.H.; Jones, R.B.; Bailey, C.J. Insulin-like effect of pinitol. Br. J. Pharmacol. 2000, 130, 1944–1948. [Google Scholar] [CrossRef] [Green Version]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. A J. Clin. Ther. 2007, 12, 259. [Google Scholar]
- Vigushin, D.M.; Poon, G.K.; Boddy, A.; English, J.; Halbert, G.W.; Pagonis, C.; Jarman, M.; Coombes, R.C. Phase I and pharmacokinetic study of d -limonene in patients with advanced cancer. Cancer Chemother Pharmacol. 1998, 42, 111–117. [Google Scholar] [CrossRef]
- Williamson, E.M. Phytocomplexes Versus Single-Entity Drugs; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Buriani, A.; Fortinguerra, S.; Carrara, M.; Pelkonen, O.; Duez, P.; Vuorela, P.M.; Vuorela, H. Systems Network Pharmaco-Toxicology in the Study of Herbal Medicines. In Toxicology of Herbal Products; Springer Science and Business Media LLC.: New York, NY, USA, 2017. [Google Scholar]
- Buriani, A. The Systems Biology Oriented, Holistic Vision of Personalized Medicine and The Emerging Concept of Proactive Herbal Medicine. J. J. Intern. Med. 2015, 1, 007. [Google Scholar]
- Schmidt, B.; Ribnicky, D.M.; Poulev, A.; Logendra, S.; Cefalu, W.T.; Raskin, I. A natural history of botanical therapeutics. Metabolism 2008, 57, S3–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskin, I.; Ripoll, C. Can an apple a day keep the doctor away? Curr. Pharm. Des. 2004, 10, 3419–3429. [Google Scholar] [CrossRef]
- Castellanos, J.R.G.; Prieto, J.M.; Heinrich, M. Red Lapacho (Tabebuia impetiginosa)—A global ethnopharmacological commodity? J. Ethnopharmacol. 2009, 121, 1–13. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Genzini, L.; Garella, D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010, 35, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Raskin, I. Health-related Interactions of Phytochemicals. J. Food Sci. 2005, 70, R20–R27. [Google Scholar] [CrossRef]
- Butler, M.S.; Buss, A.D. Natural products — The future scaffolds for novel antibiotics? Biochem. Pharmacol. 2006, 71, 919–929. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Ideker, T.; Galitski, T.; Hood, L. A new approach to decoding life: Systems biology. Annu. Rev. Genomics Hum. Genet. 2001, 2, 343–372. [Google Scholar] [CrossRef]
- Mitra, K.; Carvunis, A.-R.; Ramesh, S.K.; Ideker, T. Integrative approaches for finding modular structure in biological networks. Nat. Rev. Genet. 2013, 14, 719–732. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Yu, Y.; Wang, P.; Wang, Y.; Wang, Z.; Wang, Y. Quantitative assessment of gene expression network module-validation methods. Sci. Rep. 2015, 5, 15258. [Google Scholar] [CrossRef] [Green Version]
- Greller, L.D.; Tobin, F.L. Detecting Selective Expression of Genes and Proteins. Genome Res. 1999, 9, 282–296. [Google Scholar] [PubMed]
- Hood, L. Systems Biology and New Technologies Enable Predictive and Preventative Medicine. Science 2004, 306, 640–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csermely, P.; Korcsmáros, T.; Kiss, H.J.; London, G.; Nussinov, R. Structure and dynamics of molecular networks: A novel paradigm of drug discovery: A comprehensive review. Pharmacol. Ther. 2013, 138, 333–408. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.; Wilson, I.D. Understanding ’Global’ Systems Biology: Metabonomics and the Continuum of Metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef]
- Ghosh, S.; Matsuoka, Y.; Asai, Y.; Hsin, K.-Y.; Kitano, H. Software for systems biology: From tools to integrated platforms. Nat. Rev. Genet. 2011, 12, 821–832. [Google Scholar] [CrossRef]
- Joyce, A.R.; Palsson, B.O. The model organism as a system: Integrating ’omics’ data sets. Nat. Rev. Mol. Cell Boil. 2006, 7, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, O.; Pasanen, M.; Lindon, J.C.; Chan, K.; Zhao, L.; Deal, G.; Xu, Q.; Fan, T.-P. Omics and its potential impact on R&D and regulation of complex herbal products. J. Ethnopharmacol. 2012, 140, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Ouedraogo, M.; Baudoux, T.; Stévigny, C.; Nortier, J.; Colet, J.-M.; Efferth, T.; Qu, F.; Zhou, J.; Chan, K.; Shaw, D.; et al. Review of current and “omics” methods for assessing the toxicity (genotoxicity, teratogenicity and nephrotoxicity) of herbal medicines and mushrooms. J. Ethnopharmacol. 2012, 140, 492–512. [Google Scholar] [CrossRef]
- Buriani, A.; Garcia-Bermejo, M.L.; Bosisio, E.; Xu, Q.; Li, H.; Dong, X.; Simmonds, M.S.J.; Carrara, M.; Tejedor, N.; Lucio-Cazana, J.; et al. Omic techniques in systems biology approaches to traditional Chinese medicine research: Present and future. J. Ethnopharmacol. 2012, 140, 535–544. [Google Scholar] [CrossRef]
- Ma’Ayan, A. Introduction to Network Analysis in Systems Biology. Sci. Signal. 2011, 4, tr5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barabasi, A.-L.; Oltvai, Z.N. Network biology: Understanding the cell’s functional organization. Nat. Rev. Genet. 2004, 5, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.; Wang, X.; Xu, N.; Zhang, H.; Xu, H. Application of proteomics to determine the mechanism of action of traditional Chinese medicine remedies. J. Ethnopharmacol. 2014, 155, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitano, H. Computational systems biology. Nature 2002, 420, 206–210. [Google Scholar] [CrossRef]
- Witt, C.M.; Liu, J.; Robinson, N. Combining’omics and comparative effectiveness research: Evidence-based clinical research decision-making for Chinese medicine. Science 2015, 346, S10–S12. [Google Scholar]
- Guo, S.; Wu, J.; Zhou, W.; Liu, X.; Zhang, J.; Jia, S.; Meng, Z.; Liu, S.; Lin, R.; Liu, Y. Investigating the multi-target pharmacological mechanism of danhong injection acting on unstable angina by combined network pharmacology and molecular docking. BMC Complement. Med. Ther. 2020, 20, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yi, F.; Li, L.; Xu, L.; Meng, H.; Dong, Y.; Liu, H.-B.; Xiao, P. In silico approach in reveal traditional medicine plants pharmacological material basis. Chin. Med. 2018, 13, 33. [Google Scholar] [CrossRef] [Green Version]
- Gorgulla, C.; Boeszoermenyi, A.; Wang, Z.-F.; Fischer, P.D.; Coote, P.; Das, K.M.P.; Malets, Y.S.; Radchenko, D.S.; Moroz, Y.S.; Scott, D.A.; et al. An open-source drug discovery platform enables ultra-large virtual screens. Nature 2020, 1–8. [Google Scholar] [CrossRef]
- Wei, P.-L.; Gu, H.; Liu, J.; Wang, Z. Development of Fangjiomics for Systems Elucidation of Synergistic Mechanism Underlying Combination Therapy. Comput. Struct. Biotechnol. J. 2018, 16, 565–572. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, B.; Chen, S.; Lin, M.; Chen, Y.; Jin, S.; Chen, W.; Zhang, Y. Applications of Network Pharmacology in Traditional Chinese Medicine Research. Evid. Based Complement. Altern. Med. 2020, 2020, 1646905. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Acqua, S.D.; Innocenti, G.; Montopoli, M.; Gabbia, D.; Carrara, M. Human Adenocarcinoma Cell Line Sensitivity to Essential Oil Phytocomplexes from Pistacia Species: A Multivariate Approach. Molecules 2017, 22, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, S.; Abdollahi, M.; Saeidnia, S. Personalized medicine: A confluence of traditional and contemporary medicine. Altern. Ther. Heal. Med. 2014, 20. [Google Scholar]
- Wang, S.; Penchala, S.; Prabhu, S.; Wang, J.; Huang, Y. Molecular basis of traditional Chinese medicine in cancer chemoprevention. Curr. Drug Discov. Technol. 2010, 7, 67–75. [Google Scholar] [CrossRef]
- Lee, J.H.; Shu, L.; Fuentes, F.; Su, Z.-Y.; Kong, A.-N.T. Cancer Chemoprevention by Traditional Chinese Herbal Medicine and Dietary Phytochemicals: Targeting Nrf2-Mediated Oxidative Stress/Anti-Inflammatory Responses, Epigenetics, and Cancer Stem Cells. J. Tradit. Complement. Med. 2013, 3, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xiong, X. Current Situation and Perspectives of Clinical Study in Integrative Medicine in China. Evid. Based Complement. Altern. Med. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Chen, K.-J. Making evidence-based decisions in the clinical practice of integrative medicine. Chin. J. Integr. Med. 2010, 16, 483–485. [Google Scholar] [CrossRef]
- Chiappelli, F.; Prolo, P.; Cajulis, O.S. Evidence-based Research in Complementary and Alternative Medicine I: History. Evid. Based Complement. Altern. Med. 2005, 2, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.; Kim, K.; Nam, H.; Lee, D.S. Discovering Health Benefits of Phytochemicals with Integrated Analysis of the Molecular Network, Chemical Properties and Ethnopharmacological Evidence. Nutrients 2018, 10, 1042. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Pei, J. Chinese Herbal Medicine Meets Biological Networks of Complex Diseases: A Computational Perspective. Evid. Based Complement. Altern. Med. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Barlow, D.; Buriani, A.; Ehrman, T.; Bosisio, E.; Eberini, I.; Hylands, P. In-silico studies in Chinese herbal medicines’ research: Evaluation of in-silico methodologies and phytochemical data sources, and a review of research to date. J. Ethnopharmacol. 2012, 140, 526–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afendi, F.M.; Ono, N.; Nakamura, Y.; Nakamura, K.; Darusman, L.K.; Kibinge, N.; Morita, A.H.; Tanaka, K.; Horai, H.; Amin, A.U.; et al. DATA MINING METHODS FOR OMICS AND KNOWLEDGE OF CRUDE MEDICINAL PLANTS TOWARD BIG DATA BIOLOGY. Comput. Struct. Biotechnol. J. 2013, 4, e201301010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buriani, A.; Fortinguerra, S.; Carrara, M.; Pelkonen, O.; Duez, P.; Vuorela, P.M.; Vuorela, H. Clinical Perspectives in Diagnostic-omics and Personalized Medicine Approach to Monitor Effectiveness and Toxicity of Phytocomplexes. In Toxicology of Herbal Products; Springer Science and Business Media LLC.: New York, NY, USA, 2017; Volume 123, pp. 385–476. [Google Scholar]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Systems Biology—A Pivotal Research Methodology for Understanding the Mechanisms of Traditional Medicine. J. Pharmacopunct. 2015, 18, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Costell, M.H.; Ancellin, N.; Bernard, R.E.; Zhao, S.; Upson, J.J.; Morgan, L.A.; Maniscalco, K.; Olzinski, A.R.; Ballard, V.L.T.; Herry, K.; et al. Comparison of Soluble Guanylate Cyclase Stimulators and Activators in Models of Cardiovascular Disease Associated with Oxidative Stress. Front. Pharmacol. 2012, 3, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Pei, M.; Zheng, C.; Li, Y.; Wang, Y.; Lu, A.; Yang, L. A Systems-Pharmacology Analysis of Herbal Medicines Used in Health Improvement Treatment: Predicting Potential New Drugs and Targets. Evid. Based Complement. Altern. Med. 2013, 2013, 1–17. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, C.; Li, Y.; Wang, Y.; Lu, A.; Yang, L. Systems pharmacology in drug discovery and therapeutic insight for herbal medicines. Brief. Bioinform. 2013, 15, 710–733. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xie, X.; Zhang, J.; Sun, G. Microemulsion Electrokinetic Chromatography in Combination with Chemometric Methods to Evaluate the Holistic Quality Consistency and Predict the Antioxidant Activity of Ixeris sonchifolia (Bunge) Hance Injection. PLoS ONE 2016, 11, e0157601. [Google Scholar] [CrossRef]
- Gao, L.; Wang, X.-D.; Niu, Y.-Y.; Duan, D.-D.; Yang, X.; Hao, J.; Zhu, C.-H.; Chen, D.; Wang, K.-X.; Qin, X.-M.; et al. Molecular targets of Chinese herbs: A clinical study of hepatoma based on network pharmacology. Sci. Rep. 2016, 6, 24944. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-J.; Liang, W.-M.; Chen, C.-J.; Tsang, H.; Chiou, J.-S.; Liu, X.; Cheng, C.-F.; Lin, T.-H.; Liao, C.-C.; Huang, S.-M.; et al. Network analysis and mechanisms of action of Chinese herb-related natural compounds in lung cancer cells. Phytomedicine 2019, 58, 152893. [Google Scholar] [CrossRef]
- Bing, Z.; Cheng, Z.; Shi, D.; Liu, X.; Tian, J.; Yao, X.; Zhang, J.; Wang, Y.; Yang, K. Investigate the mechanisms of Chinese medicine Fuzhengkangai towards EGFR mutation-positive lung adenocarcinomas by network pharmacology. BMC Complement. Altern. Med. 2018, 18, 293. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, W.; Hu, Z.; Yang, M.; Li, J.; Fan, X.; Pan, H.-F.F. A Systems Pharmacology Approach for Identifying the Multiple Mechanisms of Action of the Wei Pi Xiao Decoction for the Treatment of Gastric Precancerous Lesions. Evid. Based Complement. Altern. Med. 2019, 2019, 1562707. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Zhang, Y.; Li, S.; Tan, H.Y.; Wang, N.; Mu, S.; Hao, X.; Feng, Y. A Network Pharmacology-Based Study on the Hepatoprotective Effect of Fructus Schisandrae. Molecules 2017, 22, 1617. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.-Y.; Zheng, M.-S.; Yang, X.-J.; Sun, X.-S. Analysis of molecular networks and targets mining of Chinese herbal medicines on anti-aging. BMC Complement. Altern. Med. 2016, 16, 520. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Chen, Y.; Wang, C.; Xiao, W.; Chen, S.; Zhang, S.; Yang, L.; Li, Y. Systems Pharmacology Dissection of Multi-Scale Mechanisms of Action of Huo-Xiang-Zheng-Qi Formula for the Treatment of Gastrointestinal Diseases. Front. Pharmacol. 2019, 9, 1448. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Lao, L.-X. Emerging Applications of Metabolomics in Traditional Chinese Medicine Treating Hypertension: Biomarkers, Pathways and More. Front. Pharmacol. 2019, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-F.; Hu, A.-N.; Zan, J.-F.; Wang, P.; You, Q.-Y.; Tan, A.-H. Network Pharmacology Deciphering Mechanisms of Volatiles of Wendan Granule for the Treatment of Alzheimer’s Disease. Evid. Based Complement. Altern. Med. 2019, 2019, 7826769. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, M.; Wang, H.; Li, S.; Wang, X.; Li, Y.; Wang, D.; Li, S. Network Pharmacology to Unveil the Biological Basis of Health-Strengthening Herbal Medicine in Cancer Treatment. Cancers 2018, 10, 461. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, J.; Zhou, W.; Wang, Y.; Yang, L. Systems approaches and polypharmacology for drug discovery from herbal medicines: An example using licorice. J. Ethnopharmacol. 2013, 146, 773–793. [Google Scholar] [CrossRef]
- Ansari, S.H.; Chauhan, B.; Kalam, N.; Kumar, G. Current concepts and prospects of herbal nutraceutical: A review. J. Adv. Pharm. Technol. Res. 2013, 4, 4–8. [Google Scholar] [CrossRef]
- Sendker, J.; Sheridan, H. Composition and Quality Control of Herbal Medicines. In Toxicology of Herbal Products; Springer Science and Business Media LLC.: New York, NY, USA, 2017; Volume 13, pp. 29–65. [Google Scholar]
- Pelkonen, O.; Ahokas, J.T. Toxicokinetics of Herbal Products. In Toxicology of Herbal Products; Springer Science and Business Media LLC.: New York, NY, USA, 2017; Volume 97, pp. 67–80. [Google Scholar]
- Miladinović, D.L.; Ilić, B.S.; Mihajilov-Krstev, T.M.; Nikolić, N.D.; Miladinović, L.C.; Cvetković, O.G. Investigation of the chemical composition–antibacterial activity relationship of essential oils by chemometric methods. Anal. Bioanal. Chem. 2012, 403, 1007–1018. [Google Scholar] [CrossRef]
- Cho, D.; Kim, Y.-A.; Przytycka, T.M. Chapter 5: Network Biology Approach to Complex Diseases. PLoS Comput. Boil. 2012, 8, e1002820. [Google Scholar] [CrossRef] [Green Version]
- Ulrich-Merzenich, G.; Zeitler, H.; Jobst, D.; Panek, D.; Vetter, H.; Wagner, H. Application of the “-Omic-” technologies in phytomedicine. Phytomedicine 2007, 14, 70–82. [Google Scholar] [CrossRef]
- Fortinguerra, S.; Buriani, A.; Sorrenti, V.; Lenzi, M.; Giusti, P. Molecular network-selected pharmacogenomics in a case of bipolar spectrum disorder. Pharmacogenomics 2017, 18, 1631–1642. [Google Scholar] [CrossRef]
- Li, X.-N.; Zhang, A.; Wang, M.; Sun, H.; Liu, Z.; Qiu, S.; Zhang, T.; Wang, X.-J. Screening the active compounds of Phellodendri Amurensis cortex for treating prostate cancer by high-throughput chinmedomics. Sci. Rep. 2017, 7, 46234. [Google Scholar] [CrossRef] [Green Version]
- Fodaroni, G.; Burico, M.; Gaetano, A.; Maidecchi, A.; Pagiotti, R.; Mattoli, L.; Traldi, P.; Ragazzi, E. An integrated approach to the evaluation of a metabolomic fingerprint for a phytocomplex. Focus on artichoke [Cynara cardunculus subsp. scolymus] leaf. Nat. Prod. Commun. 2014, 9, 1934578–1400900436. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Chi, Y.-H.; Niu, M.; Zhu, Y.; Zhao, Y.-L.; Chen, Z.; Wang, J.-B.; Zhang, C.-E.; Li, J.-Y.; Wang, L.-F.; et al. Metabolomics Coupled with Multivariate Data and Pathway Analysis on Potential Biomarkers in Cholestasis and Intervention Effect of Paeonia lactiflora Pall. Front. Pharmacol. 2016, 7, 1847. [Google Scholar] [CrossRef] [Green Version]
- Agatonovic-Kustrin, S.; Kettle, C.; Morton, D.W. A molecular approach in drug development for Alzheimer’s disease. Biomed. Pharmacother. 2018, 106, 553–565. [Google Scholar] [CrossRef]
- Chen, H.-S.; Qi, S.-H.; Shen, J.-G.; Chen, H. One-Compound-Multi-Target: Combination Prospect of Natural Compounds with Thrombolytic Therapy in Acute Ischemic Stroke. Curr. Neuropharmacol. 2017, 15, 134–156. [Google Scholar] [CrossRef]
- Lambrinidis, G.; Tsantili, A. Challenges with multi-objective QSAR in drug discovery. Expert Opin. Drug Discov. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Li, M.; Luo, Z.; Peng, Z.; Cai, K. Cascade-amplification of therapeutic efficacy: An emerging opportunity in cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1555. [Google Scholar] [CrossRef]
- Scotti, L.; Monteiro, A.F.M.; Viana, J.D.O.; Junior, F.J.B.M.; Ishiki, H.; Tchouboun, E.N.; Santos, R.; Scotti, M.; Mendonca, F.J.B. Multi-Target Drugs Against Metabolic Disorders. Endocrine, Metab. Immune Disord. - Drug Targets 2019, 19, 402–418. [Google Scholar] [CrossRef]
- Tibon, N.S.; Ng, C.H.; Cheong, S.L. Current progress in antimalarial pharmacotherapy and multi-target drug discovery. Eur. J. Med. Chem. 2020, 188, 111983. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Caudullo, G.; Carrara, M. Essential Oil Phytocomplex Activity, a Review with a Focus on Multivariate Analysis for a Network Pharmacology-Informed Phytogenomic Approach. Molecules 2020, 25, 1833. https://doi.org/10.3390/molecules25081833
Buriani A, Fortinguerra S, Sorrenti V, Caudullo G, Carrara M. Essential Oil Phytocomplex Activity, a Review with a Focus on Multivariate Analysis for a Network Pharmacology-Informed Phytogenomic Approach. Molecules. 2020; 25(8):1833. https://doi.org/10.3390/molecules25081833
Chicago/Turabian StyleBuriani, Alessandro, Stefano Fortinguerra, Vincenzo Sorrenti, Giada Caudullo, and Maria Carrara. 2020. "Essential Oil Phytocomplex Activity, a Review with a Focus on Multivariate Analysis for a Network Pharmacology-Informed Phytogenomic Approach" Molecules 25, no. 8: 1833. https://doi.org/10.3390/molecules25081833
APA StyleBuriani, A., Fortinguerra, S., Sorrenti, V., Caudullo, G., & Carrara, M. (2020). Essential Oil Phytocomplex Activity, a Review with a Focus on Multivariate Analysis for a Network Pharmacology-Informed Phytogenomic Approach. Molecules, 25(8), 1833. https://doi.org/10.3390/molecules25081833