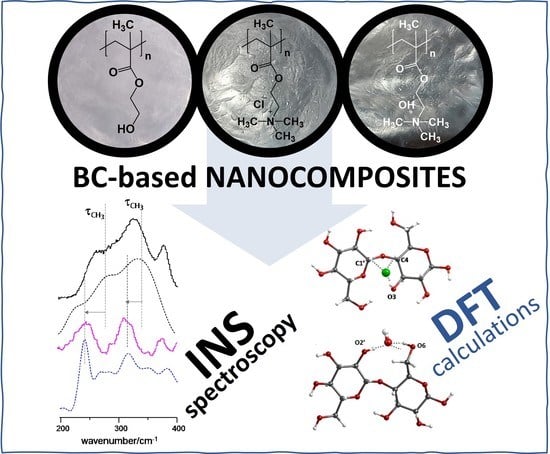
Understanding the Structure and Dynamics of Nanocellulose-Based Composites with Neutral and Ionic Poly(methacrylate) Derivatives Using Inelastic Neutron Scattering and DFT Calculations
Abstract
:1. Introduction
2. Results and Discussion
2.1. INS Spectrum of Pure Compounds
2.2. Interactions between Components in the Nanocomposites
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Nanocellulose Samples
3.3. Synthesis of Cross-Linked Polymers
3.4. Preparation of BC-Based Nanocomposites
3.5. Solid-State Carbon Cross-Polarization/Magic-Angle-Spinning Nuclear Magnetic Resonance (13C CP/MAS NMR)
3.6. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR)
3.7. Room-Temperature Fourier Transform Raman (FT-Raman)
3.8. Neutron Scattering Experiments
3.9. Spectral Analysis
3.10. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.A.A.P.; Silvestre, A.J.D.; Freire, C.S.R. Polysaccharide Based Hybrid Materials, 1st ed.; Springer: Berlin, Germany, 2018; ISBN 978-3-030-00346-3. [Google Scholar]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef]
- Figueiredo, A.R.P.; Vilela, C.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial cellulose-based nanocomposites: Roadmap for innovative materials. In Nanocellulose Polymer Composites; Thakur, V.K., Ed.; Scrivener Publishing LLC: Salem, MA, USA, 2015; pp. 17–64. [Google Scholar]
- Foresti, M.L.; Vázquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef]
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saïdi, L.; Vilela, C.; Oliveira, H.; Silvestre, A.J.D.; Freire, C.S.R. Poly(N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac. Carbohydr. Polym. 2017, 169, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, A.J.D.; Freire, C.S.R.; Neto, C.P. Do bacterial cellulose membranes have potential in drug-delivery systems? Expert Opin. Drug Deliv. 2014, 11, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Mota, J.P.; De Almeida, T.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical drug delivery systems based on bacterial nanocellulose: Accelerated stability testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Vilela, C.; Costa, E.M.; Veiga, M.; Antunes, F.; Pintado, M.M.; Sèbe, G.; Coma, V.; et al. Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J. Mol. Liq. 2020, 302, 112547. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Vilela, C.; Oliveira, H.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Nanocellulose-based antifungal nanocomposites against the polymorphic fungus Candida albicans. Carbohydr. Polym. 2019, 217, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and conductive nanocellulose-based films for active and intelligent food packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Vilela, C.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Zwitterionic nanocellulose-based membranes for organic dye removal. Materials (Basel) 2019, 12, 1404. [Google Scholar] [CrossRef] [Green Version]
- Gadim, T.D.O.; Figueiredo, A.G.P.R.; Rosero-Navarro, N.C.; Vilela, C.; Gamelas, J.A.F.; Barros-Timmons, A.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nanostructured bacterial cellulose-poly(4-styrene sulfonic acid) composite membranes with high storage modulus and protonic conductivity. ACS Appl. Mater. Interfaces 2014, 6, 7864–7875. [Google Scholar] [CrossRef]
- Vilela, C.; Cordeiro, D.M.; Boas, J.V.; Barbosa, P.; Nolasco, M.; Vaz, P.D.; Rudić, S.; Ribeiro-Claro, P.; Silvestre, A.J.D.; Oliveira, V.B.; et al. Poly(4-styrene sulfonic acid)/bacterial cellulose membranes: Electrochemical performance in a single-chamber microbial fuel cell. Bioresour. Technol. Rep. 2020, 9, 100376. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Mashkour, M.; Rahimnejad, M.; Mashkour, M. Bacterial cellulose-polyaniline nano-biocomposite: A porous media hydrogel bioanode enhancing the performance of microbial fuel cell. J. Power Sources 2016, 325, 322–328. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Vilela, C.; Loureiro, F.J.A.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nafion® and nanocellulose: A partnership for greener polymer electrolyte membranes. Ind. Crops Prod. 2016, 93, 212–218. [Google Scholar] [CrossRef]
- Padrão, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Dourado, F. Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocoll. 2016, 58, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Silva, A.C.Q.; Domingues, E.M.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C.S.R. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhou, X.; Deng, W.; Zheng, Z.; Liu, Z. Freestanding bacterial cellulose-graphene oxide composite membranes with high mechanical strength for selective ion permeation. Sci. Rep. 2016, 6, 33185. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Kokabi, M.; Mousavi, S.M. Conductive bacterial cellulose/multiwall carbon nanotubes nanocomposite aerogel as a potentially flexible lightweight strain sensor. Carbohydr. Polym. 2018, 201, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Buyanov, A.L.; Gofman, I.V.; Revel’skaya, L.G.; Khripunov, A.K.; Tkachenko, A.A. Anisotropic swelling and mechanical behavior of composite bacterial cellulose-poly(acrylamide or acrylamide-sodium acrylate) hydrogels. J. Mech. Behav. Biomed. Mater. 2010, 3, 102–111. [Google Scholar] [CrossRef]
- Amin, M.C.I.M.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo- and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473. [Google Scholar] [CrossRef]
- Hobzova, R.; Duskova-Smrckova, M.; Michalek, J.; Karpushkin, E.; Gatenholm, P. Methacrylate hydrogels reinforced with bacterial cellulose. Polym. Int. 2012, 61, 1193–1201. [Google Scholar] [CrossRef]
- Figueiredo, A.R.P.; Figueiredo, A.G.P.R.; Silva, N.H.C.S.; Barros-Timmons, A.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Antimicrobial bacterial cellulose nanocomposites prepared by in situ polymerization of 2-aminoethyl methacrylate. Carbohydr. Polym. 2015, 123, 443–453. [Google Scholar] [CrossRef]
- Vilela, C.; Gadim, T.D.O.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nanocellulose/poly(methacryloyloxyethyl phosphate) composites as proton separator materials. Cellulose 2016, 23, 3677–3689. [Google Scholar] [CrossRef]
- Vilela, C.; Martins, A.P.C.; Sousa, N.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Poly(bis[2-(methacryloyloxy)ethyl] phosphate)/bacterial cellulose nanocomposites: Preparation, characterization and application as polymer electrolyte membranes. Appl. Sci. 2018, 8, 1145. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.C.H.; Parker, S.F.; Ramirez-Cuesta, A.J.; Tomkinson, J. Vibrational Spectroscopy with Neutrons: With Applications in Chemistry, Biology, Materials Science and Catalysis; Finney, J.L., Worcester, D.L., Eds.; World Scientific Publishing Co.: Singapore, 2005; ISBN 978-981-256-013-1. [Google Scholar]
- Harrelson, T.F.; Cheng, Y.Q.; Li, J.; Jacobs, I.E.; Ramirez-Cuesta, A.J.; Faller, R.; Moulé, A.J. Identifying atomic scale structure in undoped/doped semicrystalline P3HT using Inelastic Neutron Scattering. Macromolecules 2017, 50, 2424–2435. [Google Scholar] [CrossRef] [Green Version]
- Nolasco, M.M.; Araujo, C.F.; Thiyagarajan, S.; Rudić, S.; Vaz, P.D.; Silvestre, A.J.D.; Ribeiro-Claro, P.J.A.; Sousa, A.F. Asymmetric monomer, amorphous polymer structure–property relationships in 2,4-FDCA and 2,4-PEF. Macromolecules 2020, 53, 1380–1387. [Google Scholar] [CrossRef]
- Barroso-Bujans, F.; Fernandez-Alonso, F.; Cerveny, S.; Arrese-Igor, S.; Alegría, A.; Colmenero, J. Two-dimensional subnanometer confinement of ethylene glycol and poly(ethylene oxide) by Neutron Spectroscopy: Molecular size effects. Macromolecules 2012, 45, 3137–3144. [Google Scholar] [CrossRef]
- Müller, M.; Vogl, G.; Schober, H.; Chanzy, H.; Heux, L. Inelastic Neutron Scattering study on different types of celulose. In Biological Macromolecular Dynamics; Cusack, S., Büttner, H., Ferrand, M., Langan, P., Timmins, P., Eds.; Adenine Press: New York, NY, USA, 1997. [Google Scholar]
- Müller, M.; Czihak, C.; Schober, H.; Nishiyama, Y.; Vogl, G. All disordered regions of native cellulose show common low-frequency dynamics. Macromolecules 2000, 33, 1834–1840. [Google Scholar] [CrossRef]
- Araujo, C.; Freire, C.S.R.; Nolasco, M.M.; Ribeiro-Claro, P.J.A.; Rudić, S.; Silvestre, A.J.D.; Vaz, P.D. Hydrogen bond dynamics of cellulose through Inelastic Neutron Scattering Spectroscopy. Biomacromolecules 2018, 19, 1305–1313. [Google Scholar] [CrossRef]
- Parker, S.F.; Fernandez-Alonso, F.; Ramirez-Cuesta, A.J.; Tomkinson, J.; Rudic, S.; Pinna, R.S.; Gorini, G.; Fernandez Castanon, J. Recent and future developments on TOSCA at ISIS. J. Phys. Conf. Ser. 2014, 554, 012003. [Google Scholar] [CrossRef]
- Parker, S.F.; Ramirez-Cuesta, A.J.; Daemen, L. Vibrational spectroscopy with neutrons: Recent developments. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Pinna, R.S.; Rudić, S.; Capstick, M.J.; McPhail, D.J.; Pooley, D.E.E.; Howells, G.D.; Gorini, G.; Fernandez-Alonso, F. Detailed characterisation of the incident neutron beam on the TOSCA spectrometer. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2017, 870, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Pinna, R.S.; Rudić, S.; Parker, S.F.; Armstrong, J.; Zanetti, M.; Škoro, G.; Waller, S.P.; Zacek, D.; Smith, C.A.; Capstick, M.J.; et al. The neutron guide upgrade of the TOSCA spectrometer. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 896, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, A.G.P.R.; Figueiredo, A.R.P.; Alonso-Varona, A.; Fernandes, S.C.M.; Palomares, T.; Rubio-Azpeitia, E.; Barros-Timmons, A.; Silvestre, A.J.D.; Neto, C.P.; Freire, C.S.R. Biocompatible Bacterial Cellulose-Poly(2-hydroxyethyl methacrylate) Nanocomposite Films. Biomed Res. Int. 2013, 2013, 698141. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, N.; Pinto, R.J.B.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Exploiting poly(ionic liquids) and nanocellulose for the development of bio-based anion-exchange membranes. Biomass Bioenergy 2017, 100, 116–125. [Google Scholar] [CrossRef]
- Araujo, C.F.; Coutinho, J.A.P.; Nolasco, M.M.; Parker, S.F.; Ribeiro-Claro, P.J.A.; Rudić, S.; Soares, B.I.G.; Vaz, P.D. Inelastic neutron scattering study of reline: Shedding light on the hydrogen bonding network of deep eutectic solvents. Phys. Chem. Chem. Phys. 2017, 19, 17998–18009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petridis, L.; O’Neill, H.M.; Johnsen, M.; Fan, B.; Schulz, R.; Mamontov, E.; Maranas, J.; Langan, P.; Smith, J.C. Hydration Control of the Mechanical and Dynamical Properties of Cellulose. Biomacromolecules 2014, 15, 4152–4159. [Google Scholar] [CrossRef] [PubMed]
- Malm, E.; Bulone, V.; Wickholm, K.; Larsson, P.T.; Iversen, T. The surface structure of well-ordered native cellulose fibrils in contact with water. Carbohydr. Res. 2010, 345, 97–100. [Google Scholar] [CrossRef]
- Newman, R.H.; Davidson, T.C. Molecular conformations at the cellulose–water interface. Cellulose 2004, 11, 23–32. [Google Scholar] [CrossRef]
- Asthagiri, D.; Pratt, L.R.; Kress, J.D.; Gomez, M.A. The hydration state of HO−(aq). Chem. Phys. Lett. 2003, 380, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Gluconacetobacter sacchari: An efficient bacterial cellulose cell-factory. Carbohydr. Polym. 2011, 86, 1417–1420. [Google Scholar] [CrossRef]
- Ribeiro-Claro, P.; Vilela, C.; Nolasco, M.; Rudic, S.; Vaz, P.D.; Araujo, C. Structural characterization of cellulose-based nanocomposites. STFC ISIS Neutron Muon Source 2018. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02 2009; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Walker, M.; Harvey, A.J.A.; Sen, A.; Dessent, C.E.H. Performance of M06, M06-2X, and M06-HF Density Functionals for Conformationally Flexible Anionic Clusters: M06 Functionals Perform Better than B3LYP for a Model System with Dispersion and Ionic Hydrogen-Bonding Interactions. J. Phys. Chem. A 2013, 117, 12590–12600. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathi, R.; Bellesia, G.; Chundawat, S.P.S.; Dale, B.E.; Langan, P.; Gnanakaran, S. Insights into Hydrogen Bonding and Stacking Interactions in Cellulose. J. Phys. Chem. A 2011, 115, 14191–14202. [Google Scholar] [CrossRef] [PubMed]
- Martínez González, M.; Xavier, F.G.D.; Li, J.; Montero-Cabrera, L.A.; Garcia De La Vega, J.M.; Varandas, A.J.C. Role of Augmented Basis Sets and Quest for ab Initio Performance/Cost Alternative to Kohn-Sham Density Functional Theory. J. Phys. Chem. A 2020, 124, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Cuesta, A.J. aCLIMAX 4.0.1, the new version of the software for analyzing and interpreting INS spectra. Comput. Phys. Commun. 2004, 157, 226–238. [Google Scholar] [CrossRef]
Sample Availability: Not available. |









| Nanocomposites | Nominal Composition | Measured Composition | |
|---|---|---|---|
| WBC/Wmonomer | Wpolymer/Wtotal | WBC/Wtotal | |
| BC•PHEMA | 1:2 | 0.63 ± 0.02 | 0.37 ± 0.02 |
| 1:4 | 0.86 ± 0.04 | 0.14 ± 0.04 | |
| BC•PMACC | 1:2 | 0.42 ± 0.06 | 0.58 ± 0.06 |
| 1:4 | 0.70 ± 0.05 | 0.30 ± 0.05 | |
| BC•PMACH | 1:4 | 0.70 ± 0.05 | 0.30 ± 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, C.; Freire, C.S.R.; Araújo, C.; Rudić, S.; Silvestre, A.J.D.; Vaz, P.D.; Ribeiro-Claro, P.J.A.; Nolasco, M.M. Understanding the Structure and Dynamics of Nanocellulose-Based Composites with Neutral and Ionic Poly(methacrylate) Derivatives Using Inelastic Neutron Scattering and DFT Calculations. Molecules 2020, 25, 1689. https://doi.org/10.3390/molecules25071689
Vilela C, Freire CSR, Araújo C, Rudić S, Silvestre AJD, Vaz PD, Ribeiro-Claro PJA, Nolasco MM. Understanding the Structure and Dynamics of Nanocellulose-Based Composites with Neutral and Ionic Poly(methacrylate) Derivatives Using Inelastic Neutron Scattering and DFT Calculations. Molecules. 2020; 25(7):1689. https://doi.org/10.3390/molecules25071689
Chicago/Turabian StyleVilela, Carla, Carmen S. R. Freire, Catarina Araújo, Svemir Rudić, Armando J. D. Silvestre, Pedro D. Vaz, Paulo J. A. Ribeiro-Claro, and Mariela M. Nolasco. 2020. "Understanding the Structure and Dynamics of Nanocellulose-Based Composites with Neutral and Ionic Poly(methacrylate) Derivatives Using Inelastic Neutron Scattering and DFT Calculations" Molecules 25, no. 7: 1689. https://doi.org/10.3390/molecules25071689
APA StyleVilela, C., Freire, C. S. R., Araújo, C., Rudić, S., Silvestre, A. J. D., Vaz, P. D., Ribeiro-Claro, P. J. A., & Nolasco, M. M. (2020). Understanding the Structure and Dynamics of Nanocellulose-Based Composites with Neutral and Ionic Poly(methacrylate) Derivatives Using Inelastic Neutron Scattering and DFT Calculations. Molecules, 25(7), 1689. https://doi.org/10.3390/molecules25071689