Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine
Abstract
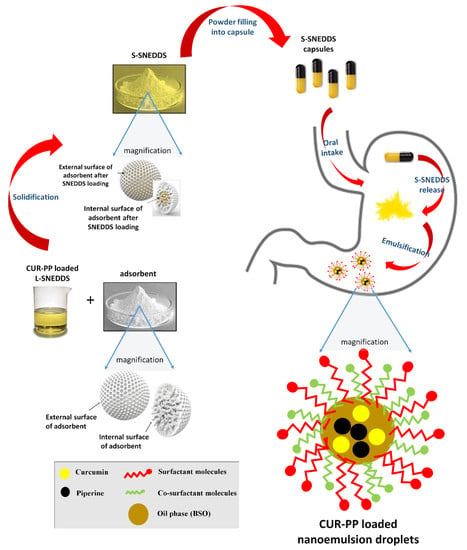
:1. Introduction
2. Results
2.1. UHPLC Analysis for CUR and PP
2.2. Characterization of Liquid CUR-PP SNEDDS
2.2.1. Equilibrium Solubility of CUR and PP in SEDDS/SNEDDS Formulations
2.2.2. Appearance and Homogeneity
2.2.3. Droplet Size and Zeta Potential
2.2.4. Dynamic Dispersion Studies
2.3. Characterization of CUR-PP Solid SNEDDS
2.3.1. Scanning Electron Microscopy
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. X-Ray Powder Diffraction (XRPD)
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. In-Vitro Dissolution Study
2.4.1. Influence of SNEDDS
2.4.2. Influence of pH
2.4.3. Influence of Adsorbent
3. Discussion
- (a)
- The high proportion of hydrophilic excipients in the formulation F6 (Type IIIB system) [15].
- (b)
- (c)
- The inclusion of the water soluble cosolvent TcP in the formulation.
- (d)
- The inclusion of the highly hydrophilic surfactant Cr-RH40 that has higher HLB (14–16) compared to HCO-40 (12.5) and T85 (11).
4. Materials and Methods
4.1. Plant Material
4.1.1. Black Seed Oil (BSO)
Seed Collection and Extraction
BSO Standardization
4.1.2. Zanthoxylum Rhetsa Seed Oil (ZRO)
Seed Collection
Extraction and Isolation
4.2. Chemicals and Reagents
4.3. UHPLC Analysis for CUR and PP Quantification
4.4. Preparation of the CUR–PP-SNEDDS Formulation
4.5. Characterization of Liquid CUR–PP SNEDDS Formulation
4.5.1. Equilibrium Solubility of CUR and PP in SNEDDS Formulations
4.5.2. Appearance and Morphology
4.5.3. Droplet Size and Zeta Potential
4.5.4. Dynamic Dispersion Studies
4.6. Solidification of CUR-PP Loaded Liquid SNEDDS
4.7. Characterization of Solid CUR-PP Loaded SNEDDS
4.7.1. Scanning Electron Microscopy (SEM)
4.7.2. Differential Scanning Calorimetry (DSC)
4.7.3. X-Ray Powder Diffraction (XRPD)
4.7.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.8. In-Vitro Dissolution Tests
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naik, R.R.; Shakya, A.K.; Khalaf, N.A.; Abuhamdah, S.; Oriquat, G.A.; Maraqa, A. GC-MS Analysis and Biological Evaluation of Essential Oil of Zanthoxylum Rhesta (Roxb.) DC Pericarp. Jordan J. Pharm. Sci. 2015, 8, 181–193. [Google Scholar] [CrossRef]
- de Holanda Cavalcanti, S.C.; de Oliveira, R.D.R.B.; de Sousa, D.P. Antitumor Essential Oils: Progress in Medicinal Chemistry. In Bioactive Essential Oils and Cancer; de Sousa, D.P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 111–124. [Google Scholar]
- Cheng, K.-W.; Wong, C.C.; Mattheolabakis, G.; Xie, G.; Huang, L.; Rigas, B. Curcumin enhances the lung cancer chemopreventive efficacy of phospho-sulindac by improving its pharmacokinetics. Int. J. Oncol. 2013, 43, 895–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, J.; Moore-Medlin, T.; Sonavane, K.; Ekshyyan, O.; McLarty, J.; Nathan, C.-A.O. Curcumin inhibits UV radiation–induced skin cancer in SKH-1 mice. Otolaryngol. Head Neck Surg. 2013, 148, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; McEachern, M.D.; Shah, S.H.; Rong, Y.; Rong, X.; Smelley, C.L.; Caldito, G.C.; Abreo, F.W.; Nathan, C. Curcumin inhibits carcinogen and nicotine-induced Mammalian target of rapamycin pathway activation in head and neck squamous cell carcinoma. Cancer Prev. Res. 2010, 3, 1586–1595. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-C.; Chen, H.-W.; Kuo, Y.-C.; Chang, Y.-F.; Lee, Y.-J.; Hwang, J.-J. Therapeutic efficacy evaluation of curcumin on human oral squamous cell carcinoma xenograft using multimodalities of molecular imaging. Am. J. Chin. Med. 2010, 38, 343–358. [Google Scholar] [CrossRef]
- Yoysungnoen, P.; Wirachwong, P.; Bhattarakosol, P.; Niimi, H.; Patumraj, S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin. Hemorheol. Microcirc. 2006, 34, 109–115. [Google Scholar]
- Huang, M.-T.; Lou, Y.-R.; Xie, J.G.; Ma, W.; Lu, Y.-P.; Yen, P.; Zhu, B.T.; Newmark, H.; Ho, C.-T. Effect of dietary curcumin and dibenzoylmethane on formation of 7, 12-dimethylbenz [a] anthracene-induced mammary tumors and lymphomas/leukemias in Sencar mice. Carcinogenesis 1998, 19, 1697–1700. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Cui, J.; Yu, B.; Zhao, Y.; Zhu, W.; Li, H.; Lou, H.; Zhai, G. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int. J. Pharm. 2009, 371, 148–155. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Alhasani, K.F.; Kazi, M.; Ibrahim, M.A.; Shahba, A.A.; Alanazi, F.K. Self-nanoemulsifying ramipril tablets: A novel delivery system for the enhancement of drug dissolution and stability. Int J. Nanomed. 2019, 14, 5435–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.; Sawant, K.K. Self micro-emulsifying drug delivery system: Formulation development and biopharmaceutical evaluation of lipophilic drugs. Curr. Drug Deliv. 2009, 6, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.; Porter, C. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ’self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11 (Suppl. 2), S93–S98. [Google Scholar] [CrossRef]
- Shao, B.; Cui, C.; Ji, H.; Tang, J.; Wang, Z.; Liu, H.; Qin, M.; Li, X.; Wu, L. Enhanced oral bioavailability of piperine by self-emulsifying drug delivery systems: In vitro, in vivo and in situ intestinal permeability studies. Drug Deliv. 2015, 22, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Setthacheewakul, S.; Mahattanadul, S.; Phadoongsombut, N.; Pichayakorn, W.; Wiwattanapatapee, R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur. J. Pharm. Biopharm. 2010, 76, 475–485. [Google Scholar] [CrossRef]
- Ramshankar, Y.V.; Suresh, S.; Devi, K. Novel Self-emulsifying Formulation of Curcumin with Improved Dissolution, Antiangiogenic and Anti-inflammatory Activity. Clin. Res. Regul. Aff. 2008, 25, 213–234. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and liver disease: From chemistry to medicine. Compr. Rev. Food Sci. Food Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, C.; Lin, Z.; Guo, Y.; Shi, L.; Dong, P.; Lu, Z.; Gao, S.; Liao, Y.; Chen, B. Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation–a novel mechanism suppressing liver fibrosis. FEBS J. 2014, 281, 88–103. [Google Scholar] [CrossRef]
- Choudhary, K.M.; Mishra, A.; Poroikov, V.V.; Goel, R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur. J. Pharmacol. 2013, 704, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sanmukhani, J.; Satodia, V.; Trivedi, J.; Patel, T.; Tiwari, D.; Panchal, B.; Goel, A.; Tripathi, C.B. Efficacy and safety of curcumin in major depressive disorder: A randomized controlled trial. Phytother. Res. 2014, 28, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, J.; Zhang, M.; Yao, W.; Ma, X.; Yu, S.Y. Effects of curcumin on chronic, unpredictable, mild, stress-induced depressive-like behaviour and structural plasticity in the lateral amygdala of rats. Int. J. Neuropsychopharmacol. 2014, 17, 793–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.-C.; Zhang, B.; Liao, M.-J.; Zhang, W.-X.; He, W.-Y.; Wang, H.-B.; Yang, C.-X. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci. Lett. 2014, 560, 81–85. [Google Scholar] [CrossRef]
- Yu, C.-W.; Wei, C.-C.; Liao, V.-C. Curcumin-mediated oxidative stress resistance in Caenorhabditis elegans is modulated by age-1, akt-1, pdk-1, osr-1, unc-43, sek-1, skn-1, sir-2.1, and mev-1. Free Radic. Res. 2014, 48, 371–379. [Google Scholar] [CrossRef]
- Singla, V.; Pratap Mouli, V.; Garg, S.K.; Rai, T.; Choudhury, B.N.; Verma, P.; Deb, R.; Tiwari, V.; Rohatgi, S.; Dhingra, R. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis—A randomized, placebo-controlled, pilot study. J. Crohn’s Colitis 2014, 8, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.-G.; Lee, S.-Y.; Huang, Z.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-F.; Zu, J.-N.; Li, J.; Chen, C.; Xi, C.-Y.; Yan, J.-L. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci. Lett. 2014, 560, 51–56. [Google Scholar] [CrossRef]
- Qu, H.; Lv, M.; Xu, H. Piperine: Bioactivities and structural modifications. Mini Rev. Med. Chem. 2015, 15, 145–156. [Google Scholar] [CrossRef]
- Alarifi, S.; Aldahmash, B.; El-Nagar, D.; Dkhil, M. Effect of corn oil, flaxseed oil and black seed oil on lead acetate-induced hepatic tissue damage: A histological study. J. Med. Plants Res. 2012, 6, 4128–4134. [Google Scholar]
- Salem, M.L.; Hossain, M.S. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int. J. Immunopharmacol. 2000, 22, 729–740. [Google Scholar] [CrossRef]
- Hussain, D.A.S.; Hussain, M.M. Nigella sativa (black seed) is an effective herbal remedy for every disease except death—A Prophetic statement which modern scientists confirm unanimously: A review. Adv. Med. Plant. Res. 2016, 4, 27–57. [Google Scholar]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Durmaz, G.; Karabulut, İ.; Topçu, A.; Asiltürk, M.; Kutlu, T. Roasting-related changes in oxidative stability and antioxidant capacity of apricot kernel oil. J. Am. Oil Chem. Soc. 2010, 87, 401–409. [Google Scholar] [CrossRef]
- Eid, A.M.; Elmarzugi, N.A.; El-Enshasy, H.A. Development of avocado oil nanoemulsion hydrogel using sucrose ester stearate. J. Appl. Pharm. Sci. 2013, 3, 145. [Google Scholar]
- Reddy, L.J.; Jose, B. Statistical analysis of the antibacterial activity of Zanthoxylum rhetsa seed essential oil. J. Chem. Pharm. Res. 2011, 3, 440–444. [Google Scholar]
- Islam, M.; Biswas, N.N.; Saha, S.; Hossain, H.; Jahan, I.A.; Khan, T.A.; Awang, K.; Shilpi, J.A. Antinociceptive and antioxidant activity of Zanthoxylum budrunga Wall (Rutaceae) seeds. Sci. World J. 2014, 2014, 869537. [Google Scholar] [CrossRef] [Green Version]
- Naik, R.R. GC-FID analysis of fatty acids and biological activity of Zanthoxylum rhetsa seed oil. Orient. J. Chem. 2015, 31, 1929–1935. [Google Scholar] [CrossRef]
- Shahba, A.A.; Ahmed, A.R.; Mohsin, K.; Abdel-Rahman, S.I.; Alanazi, F.K. Solidification of cinnarizine self-nanoemulsifying drug delivery systems by fluid bed coating: Optimization of the process and formulation variables. Pharmazie 2017, 72, 143–151. [Google Scholar]
- Rashid, R.; Kim, D.W.; Yousaf, A.M.; Mustapha, O.; Fakhar Ud, D.; Park, J.H.; Yong, C.S.; Oh, Y.-K.; Youn, Y.S.; Kim, J.O.; et al. Comparative study on solid self-nanoemulsifying drug delivery and solid dispersion system for enhanced solubility and bioavailability of ezetimibe. Int. J. Nanomed. 2015, 10, 6147–6159. [Google Scholar]
- Lei, Y.; Lu, Y.; Qi, J.; Nie, S.; Hu, F.; Pan, W.; Wu, W. Solid self-nanoemulsifying cyclosporin A pellets prepared by fluid-bed coating: Preparation, characterization and in vitro redispersibility. Int. J. Nanomed. 2011, 6, 795–805. [Google Scholar]
- Wang, Z.; Sun, J.; Wang, Y.; Liu, X.; Liu, Y.; Fu, Q.; Meng, P.; He, Z. Solid self-emulsifying nitrendipine pellets: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2010, 383, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shahba, A.A.; Alanazi, F.K.; Mohsin, K.; Abdel-Hamid, M. Stability Assessment of Cinnarizine in Self-Emulsifying Drug Delivery Systems. Lat. Am. J. Pharm. 2012, 31, 549–554. [Google Scholar]
- Gumaste, S.G.; Dalrymple, D.M.; Serajuddin, A.T.M. Development of Solid SEDDS, V: Compaction and Drug Release Properties of Tablets Prepared by Adsorbing Lipid-Based Formulations onto Neusilin® US2. Pharm. Res. 2013, 30, 3186–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwadei, M.; Kazi, M.; Alanazi, F.K. Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals—Curcumin and thymoquinone. Saudi Pharm. J. 2019, 27, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Pouton, C.; Cuine, J.; Charman, W. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 673–691. [Google Scholar] [CrossRef]
- Tarr, B.D.; Yalkowsky, S.H. Enhanced intestinal absorption of cyclosporine in rats through the reduction of emulsion droplet size. Pharm. Res. 1989, 6, 40–43. [Google Scholar] [CrossRef]
- Shah, N.; Carvajal, M.; Patel, C.; Infeld, M.; Malick, A. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int. J. Pharm. 1994, 106, 15–23. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Chen, L.; Zhang, W. Influence of Ionic Surfactants on the Properties of Nanoemulsions Emulsified by Nonionic Surfactants Span 80/Tween 80. J. Dispers. Sci. Technol. 2016, 37, 1511–1517. [Google Scholar] [CrossRef]
- Mohsin, K. Design of lipid-based formulations for oral administration of poorly water-soluble drug fenofibrate: Effects of digestion. Aaps Pharmscitech. 2012, 13, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 9, 20091–20112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yogesh, C.; Upendra, R.; Fred, M.; Thomas, P.; Hans, H.; William, M. Comparative evaluation of porous silica based carriers for lipids and liquid drug formulations. Open Mater. Sci. 2014, 1. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Freire, B.O.S.; Serajuddin, A.T.M. Development of solid SEDDS, VI: Effect of precoating of Neusilin((R)) US2 with PVP on drug release from adsorbed self-emulsifying lipid-based formulations. Eur. J. Pharm. Sci. 2017, 110, 124–133. [Google Scholar] [CrossRef]
- Limnell, T.; Santos, H.A.; Makila, E.; Heikkila, T.; Salonen, J.; Murzin, D.Y.; Kumar, N.; Laaksonen, T.; Peltonen, L.; Hirvonen, J. Drug delivery formulations of ordered and nonordered mesoporous silica: Comparison of three drug loading methods. J. Pharm. Sci. 2011, 100, 3294–3306. [Google Scholar] [CrossRef]
- Kazi, M.; Shariare, M.H.; Al-bgomi, M.; Hussain, M.D.; Alanazi, F.K. Simultaneous determination of curcumin (Cur) and thymoquinone (THQ) in lipid based self-nanoemulsifying systems and its application to the commercial product using UHPLC-UV-Vis spectrophotometer. Curr. Pharm. Anal. 2018, 14, 277–285. [Google Scholar] [CrossRef]
- Shafi, P.M.; Saidutty, A.; Clery, R.A. Volatile Constituents of Zanthoxylum rhetsa Leaves and Seeds. J. Essent. Oil Res. 2000, 12, 179–182. [Google Scholar] [CrossRef]
- Theeramunkong, S.; Utsintong, M. Comparison between Volatile Oil from Fresh and Dried Fruits of Zanthoxylum rhetsa (Roxb.) DC. and Cytotoxicity Activity Evaluation. Pharmacogn. J. 2018, 10, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Knapik, J.; Wojnarowska, Z.; Grzybowska, K.; Jurkiewicz, K.; Stankiewicz, A.; Paluch, M. Stabilization of the Amorphous Ezetimibe Drug by Confining Its Dimension. Mol. Pharm. 2016, 13, 1308–1316. [Google Scholar] [CrossRef]
- Shahba, A.A.; Mohsin, K.; Alanazi, F.K. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: Design, optimization, and in-vitro assessment. Aaps Pharmscitech. 2012, 13, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Bi, J.; Tian, H.; Jin, Y.; Wang, Y.; Yang, X.; Yang, Z.; Kou, J.; Li, F. Preparation and evaluation of a self-nanoemulsifying drug delivery system loaded with Akebia saponin D-phospholipid complex. Int. J. Nanomed. 2016, 11, 4919–4929. [Google Scholar]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Poorly Water-Soluble Talinolol: Preparation, in vitro and in vivo Assessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Z.; Hou, X.; Jia, X.B. Design and optimization of self-nanoemulsifying drug delivery systems for improved bioavailability of cyclovirobuxine D. Drug Des. Dev. Ther. 2016, 10, 2049–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahba, A.A.-W.; Mohsin, K.; Alanazi, F.K.; Abdel-Rahman, S.I. Optimization of Self-Nanoemulsifying Formulations for Weakly Basic Lipophilic Drugs: Role of Acidification and Experimental Design. Braz. J. Pharm. Sci. 2016, 52, 653–667. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Chavhan, S.; Sawant, K.K. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 145–155. [Google Scholar] [CrossRef]
- Mohsin, K.; Long, M.A.; Pouton, C.W. Design of lipid-based formulations for oral administration of poorly water-soluble drugs: Precipitation of drug after dispersion of formulations in aqueous solution. J. Pharm. Sci. 2009, 98, 3582–3595. [Google Scholar] [CrossRef]
- Shazly, G.; Mohsin, K. Dissolution improvement of solid self-emulsifying drug delivery systems of fenofibrate using an inorganic high surface adsorption material. Acta Pharm. 2015, 65, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Shahba, A.A.-W.; Ahmed, A.R.; Alanazi, F.K.; Mohsin, K.; Abdel-Rahman, S.I. Multi-Layer Self-Nanoemulsifying Pellets: An Innovative Drug Delivery System for the Poorly Water-Soluble Drug Cinnarizine. Aaps Pharmscitech. 2018, 19, 2087–2102. [Google Scholar] [CrossRef]
- Tian, Z.; Yi, Y.; Yuan, H.; Han, J.; Zhang, X.; Xie, Y.; Lu, Y.; Qi, J.; Wu, W. Solidification of nanostructured lipid carriers (NLCs) onto pellets by fluid-bed coating: Preparation, in vitro characterization and bioavailability in dogs. Powder Technol. 2013, 247, 120–127. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, N.; Wu, B.; Lu, Y.; Guan, T.; Wu, W. Physical characterization of lansoprazole/PVP solid dispersion prepared by fluid-bed coating technique. Powder Technol. 2008, 182, 480–485. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Elzayat, E.M.; Alanazi, F.K. Development of modified in situ gelling oral liquid sustained release formulation of dextromethorphan. Drug Dev. Ind. Pharm. 2012, 38, 971–978. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds CUR and PP SNEDDS are available from the authors. |













| Ingredient | Biological Activity | References |
|---|---|---|
| CUR |
| [20,21,22,23,24,25,26,27,28,29] |
| PP |
| [30] |
| BSO |
| [31,32,33,34] |
| APO |
| [35] |
| AVO |
| [36] |
| ZRO |
| [37,38,39] |
| No | LFCS Type | Formulation (w/w) | Solubility (mg/g) | |
|---|---|---|---|---|
| CUR | PP | |||
| F1 | IIIA | APO:I988(7:3)/HCO40 [1:1] | 30.4 | 36.6 |
| F2 | IIIA | AVO:I988(7:3)/HCO40 [1:1] | 25.2 | 37.0 |
| F3 | IIIA | BSO:I988(7:3)/HCO40 [1:1] | 28.2 | 39.1 |
| F4 | IIIA | ZRO:I988(7:3)/HCO40 [1:1] | 32.5 | 44.5 |
| F5 | II | ZRO:I988(7:3)/T85 [1:1] | 19.0 | 48.2 |
| F6 | IIIB | BSO:I988:TcP(2:2:1)/CrRH40 [1:1] | 38.4 | 45.0 |
| No | LFCS Type | Formulation (w/w) | Homogeneity | Spontaneity | Appearance |
|---|---|---|---|---|---|
| F1 | IIIA | APO:I988(7:3)/HCO40 [1:1] | Yes | <1 min | Hazy |
| F2 | IIIA | AVO:I988(7:3)/HCO40 [1:1] | Yes | ~5 sec | Turbid |
| F3 | IIIA | BSO:I988(7:3)/HCO40 [1:1] | Yes | ~5 sec | Transparent |
| F4 | IIIA | ZRO:I988(7:3)/HCO40 [1:1] | Yes | <1 min | Hazy |
| F5 | II | ZRO:I988(7:3)/T85 [1:1] | Yes | <1 min | Turbid |
| F6 | IIIB | BSO:I988:TcP(2:2:1)/CrRH40 [1:1] | Yes | ~5 sec | Transparent |
| Drug | Formulation | Dissolution Efficiency (%) * |
|---|---|---|
| Curcumin | Pure Drug Powder | 0 a |
| F1A | 7.0 ± 0.4 b | |
| F1N | 35.1 ± 0.6 d | |
| F2A | 4.1 ± 0.5 e | |
| F2N | 29.4 ± 0.5 f | |
| F6A | 7.6 ± 1.6 b | |
| F6N | 45.7 ± 0.6 c | |
| Piperine | Pure Drug Power | 13.9 ± 1.7 i |
| F1A | 36.4 ± 3.8 iii | |
| F1N | 42.7 ± 1.8 iv | |
| F2A | 42.6 ± 3.8 iv | |
| F2N | 51.5 ± 4.3 v | |
| F6A | 67.8 ± 3.8 ii | |
| F6N | 65.7 ± 1.0 ii |
| Adsorbent | Aeropearl® 300 | Neusilin® US2 |
|---|---|---|
| Chemical Composition [60] | Granulated fumed silica | Magnesium aluminometasilicate |
| Chemical Formula | SiO2 | Al2O3·MgO·1.7SiO2·xH2O |
| Specific Surface Area (m2/g) [54] | 300 | 300 |
| Particle Size (µm) [60] | 30–40 | 60–120 |
| Pore Volume (ml/g) * [54] | 2.2 | 4 |
| Peak Pore Size (nm) ** [54] | ≈25 | ≈800 |
| Predominant Type of Pores [54,55] | Mesoporous (2–50nm) | Macroporous (>50nm) |
| Formulation | LFCS Type | Excipients Percentage (w/w %) | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APO | AVO | BSO | ZRO | I988 | TcP | HCO-40 | Cr-RH40 | T85 | NUS | A300 | |||
| F1 | IIIA | 35 | - | - | - | 15 | - | 50 | - | - | - | - | 100 |
| F2 | IIIA | - | 35 | - | - | 15 | - | 50 | - | - | - | - | 100 |
| F3 | IIIA | - | - | 35 | - | 15 | - | 50 | - | - | - | - | 100 |
| F4 | IIIA | - | - | - | 35 | 15 | - | 50 | - | - | - | - | 100 |
| F5 | II | - | - | - | 35 | 15 | - | - | - | 50 | - | - | 100 |
| F6 | IIIB | - | - | 20 | - | 20 | 10 | - | 50 | - | - | - | 100 |
| F1A | IIIA | 17.5 | - | - | - | 7.5 | - | 25 | - | - | - | 50 | 100 |
| F2A | IIIA | - | 17.5 | - | - | 7.5 | - | 25 | - | - | - | 50 | 100 |
| F6A | IIIB | - | - | 10 | - | 10 | 5 | - | 25 | - | - | 50 | 100 |
| F1N | IIIA | 17.5 | - | - | - | 7.5 | - | 25 | - | - | 50 | - | 100 |
| F2N | IIIA | - | 17.5 | - | - | 7.5 | - | 25 | - | - | 50 | - | 100 |
| F6N | IIIB | - | - | 10 | - | 10 | 5 | - | 25 | - | 50 | - | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine. Molecules 2020, 25, 1703. https://doi.org/10.3390/molecules25071703
Kazi M, Shahba AA, Alrashoud S, Alwadei M, Sherif AY, Alanazi FK. Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine. Molecules. 2020; 25(7):1703. https://doi.org/10.3390/molecules25071703
Chicago/Turabian StyleKazi, Mohsin, Ahmad A. Shahba, Saad Alrashoud, Majed Alwadei, Abdelrahman Y. Sherif, and Fars K. Alanazi. 2020. "Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine" Molecules 25, no. 7: 1703. https://doi.org/10.3390/molecules25071703
APA StyleKazi, M., Shahba, A. A., Alrashoud, S., Alwadei, M., Sherif, A. Y., & Alanazi, F. K. (2020). Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine. Molecules, 25(7), 1703. https://doi.org/10.3390/molecules25071703