Antibacterial Natural Halimanes: Potential Source of Novel Antibiofilm Agents
Abstract
:1. Introduction
2. Halimanes of Marine and Bacterial Origin
3. Classification
3.1. Marine Simple Halimanes, 8–14
3.2. Halimane-Glycerol Derivatives Isolated from Marine Organisms: 15–18
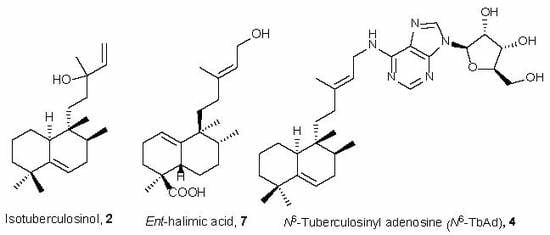
3.3. Halimane-Purines Derivatives: 19–29
3.4. Halimanes Isolated from Bacteria
3.5. Antibacterial Halimanes Isolated from Plants
4. Halimane Synthesis
4.1. Halimane-Purine Synthesis. (+)-Agelasine C
4.2. Synthesis of (+)-agelasimine A, 26, and (+)-agelasimine B, 27
4.3. Synthesis of Tuberculosinol, 1, and Isotuberculosinol, 2
4.4. Snider’s Synthesis of Tuberculosinol, 1, and Isotuberculosinol, 2
4.5. Sorensen’s Synthesis of Tuberculosinol, 1, and Isotuberculosinol, 2
4.6. Barrero´s Synthesis of Isotuberculosinol, 2
4.7. Tuberculosinyl Adenosine (1-TbAd), 3, and N6-Tuberculosinyl Adenosine (N6-TbAd), 4, Syntheses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Esteban, J.; García-Coca, M. Mycobacterium Biofilms. Front. Microbiol. 2018, 8, 2651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M.; Costerton, J.W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, D.G. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial biofilm eradication agents: a current review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lienhardt, C.; Glaziou, P.; Uplekar, M.; Lönnroth, K.; Getahun, H.; Raviglione, M. Global tuberculosis control: lessons learnt and future prospects. Nat. Rev. Microbiol. 2012, 10, 407–416. [Google Scholar] [CrossRef]
- Anderson, G.G.; O’Toole, G.A. Bacterial Biofilms; Romeo, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 85–105. ISBN 978-3-642-09469-9. [Google Scholar]
- Lewis, K. Bacterial Biofilms; Romeo, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 107–131. ISBN 978-3-642-09469-9. [Google Scholar]
- Kester, J.C.; Fortune, S.M. Persisters and beyond: Mechanisms of phenotypic drug resistance and drug tolerance in bacteria. Crit. Rev. Biochem. Mol. Boil. 2013, 49, 91–101. [Google Scholar] [CrossRef]
- Liu, C.-I.; Liu, G.Y.; Song, Y.; Yin, F.; Hensler, M.E.; Jeng, W.-Y.; Nizet, V.; Wang, A.H.-J.; Oldfield, E. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science 2008, 319, 1391–1394. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, T.; Nakano, C.; Ootsuka, T.; Shinohara, Y.; Hara, T. Substrate specificity of Rv3378c, an enzyme from Mycobacterium tuberculosis, and the inhibitory activity of the bicyclic diterpenoids against macrophage phagocytosis. Org. Biomol. Chem. 2011, 9, 2156–2165. [Google Scholar] [CrossRef]
- Sato, T.; Kigawa, A.; Takagi, R.; Adachi, T.; Hoshino, T. Biosynthesis of a novel cyclic C35-terpene via the cyclisation of a Z-type C35-polyprenyl diphosphate obtained from a nonpathogenic Mycobacterium species. Org. Biomol. Chem. 2008, 6, 3788–3794. [Google Scholar] [CrossRef]
- Nakano, C.; Oshima, M.; Kurashima, N.; Hoshino, T. Identification of a new diterpene biosynthetic gene cluster that produces O-methylkolavelool in Herpetosiphon aurantiacus. ChemBioChem 2015, 16, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Mann, F.M.; Prisic, S.; Hu, H.; Xu, M.; Coates, R.M.; Peters, R.J. Characterization and inhibition of a class II diterpene cyclase from Mycobacterium tuberculosis: implications for tuberculosis. J. Boil. Chem. 2009, 284, 23574–23579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, F.M.; VanderVen, B.; Peters, R.J. Magnesium depletion triggers production of an immune modulating diterpenoid in Mycobacterium tuberculosis. Mol. Microbiol. 2011, 79, 1594–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prach, L.; Kirby, J.; Keasling, J.D.; Alber, T. Diterpene production in Mycobacterium tuberculosis. FEBS J. 2010, 277, 3588–3595. [Google Scholar] [CrossRef] [PubMed]
- Nakano, C.; Ootsuka, T.; Takayama, K.; Mitsui, T.; Sato, T.; Hoshino, T. Characterization of the Rv3378c gene product, a new diterpene synthase for producing tuberculosinol and (13 R,S)-isotuberculosinol (nosyberkol), from the Mycobacterium tuberculosis H37Rv genome. Biosci. Biotechnol. Biochem. 2011, 75, 75–81. [Google Scholar] [CrossRef]
- Mann, F.M.; Xu, M.; Chen, X.; Fulton, D.B.; Russell, D.G.; Peters, R.J. Edaxadiene: A new bioactive diterpene from Mycobacterium tuberculosis. J. Am. Chem. Soc. 2009, 131, 17526–17527. [Google Scholar] [CrossRef] [Green Version]
- Young, D.C.; Layre, E.; Pan, S.-J.; Tapley, A.; Adamson, J.; Seshadri, C.; Wu, Z.; Buter, J.; Minnaard, A.J.; Coscolla, M.; et al. In vivo biosynthesis of terpene nucleosides provides unique chemical markers of Mycobacterium tuberculosis infection. Chem. Boil. 2015, 22, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Buter, J.; Heijnen, D.; Wan, I.C.; Bickelhaupt, F.M.; Young, D.C.; Otten, E.; Moody, D.B.; Minnaard, A.J. Stereoselective synthesis of 1-tuberculosinyl adenosine; a virulence factor of Mycobacterium tuberculosis. J. Org. Chem. 2016, 81, 6686–6696. [Google Scholar] [CrossRef]
- Layre, E.; Lee, H.J.; Young, D.C.; Martinot, A.J.; Buter, J.; Minnaard, A.J.; Annand, J.W.; Fortune, S.M.; Snider, B.B.; Matsunaga, I.; et al. Molecular profiling of Mycobacterium tuberculosis identifies tuberculosinyl nucleoside products of the virulence-associated enzyme Rv3378c. Proc. Natl. Acad. Sci. USA 2014, 111, 2978–2983. [Google Scholar] [CrossRef] [Green Version]
- Roncero, A.M.; Tobal, I.E.; Moro, R.F.; Díez, D.; Marcos, I.S. Halimane diterpenoids: sources, structures, nomenclature and biological activities. Nat. Prod. Rep. 2018, 35, 955–991. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.; Kurabayashi, M.; Nagahori, H.; Ogiso, A.; Mishima, H. Chettaphanin-I, a novel furanoditerpenoid. Tetrahedron Lett. 1970, 11, 1095–1098. [Google Scholar] [CrossRef]
- Sato, A.; Kurabayashi, M.; Ogiso, A.; Mishima, H. Chettaphanin-II, a novel furanoditerpenoid. Tetrahedron Lett. 1971, 12, 839–842. [Google Scholar] [CrossRef]
- Urones, J.G.; Marcos, I.S.; Basabe, P.; Sexmero, M.J.; Carrillo, H.; Melchor, M.J. Minor diterpenoids from Halimium viscosum. Phytochemistry 1994, 37, 1359–1361. [Google Scholar] [CrossRef]
- Mann, F.M.; Peters, R.J. Isotuberculosinol: the unusual case of an immunomodulatory diterpenoid from Mycobacterium tuberculosis. MedChemComm 2012, 3, 899–904. [Google Scholar] [CrossRef] [Green Version]
- Nakano, C.; Okamura, T.; Sato, T.; Dairi, T.; Hoshino, T. Mycobacterium tuberculosis H37Rv3377c encodes the diterpene cyclase for producing the halimane skeleton. Chem. Commun. 2005, 1016–1018. [Google Scholar] [CrossRef]
- Nakano, C.; Hoshino, T.; Sato, T.; Toyomasu, T.; Dairi, T.; Sassa, T. Substrate specificity of the CYC2 enzyme from Kitasatospora griseola: production of sclarene, biformene, and novel bicyclic diterpenes by the enzymatic reactions of labdane- and halimane-type diterpene diphosphates. Tetrahedron Lett. 2010, 51, 125–128. [Google Scholar] [CrossRef]
- Spangler, J.E.; Carson, C.A.; Sorensen, E.J. Synthesis enables a structural revision of the Mycobacterium tuberculosis-produced diterpene, edaxadiene. Chem. Sci. 2010, 1, 202–205. [Google Scholar] [CrossRef] [Green Version]
- Urones, J.G.; Teresa, J.D.P.; Marcos, I.S.; Martín, D.D.; Garrido, N.M.; Guerra, R. Diterpenoids from halimium viscosum. Phytochemistry 1987, 26, 1077–1079. [Google Scholar] [CrossRef]
- Marcos, I.S.; Hernández, F.; Sexmero, M.; Díez, D.; Basabe, P.; Pedrero, A.; García, N.; Urones, J.G. Synthesis and absolute configuration of (−)-chettaphanin I and (−)-chettaphanin II. Tetrahedron 2003, 59, 685–694. [Google Scholar] [CrossRef]
- Rudi, A.; Aknin, M.; Gaydou, E.; Kashman, Y. Asmarines I, J, and K and nosyberkol: four new compounds from the marine sponge Raspailia sp. J. Nat. Prod. 2004, 67, 1932–1935. [Google Scholar] [CrossRef]
- Maugel, N.; Mann, F.M.; Hillwig, M.; Peters, R.J.; Snider, B.B. Synthesis of (±)-nosyberkol (isotuberculosinol, revised structure of edaxadiene) and (±)-tuberculosinol. Org. Lett. 2010, 12, 2626–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciavatta, M.L.; García-Matucheski, S.; Carbone, M.; Villani, G.; Nicotera, M.R.; Muniain, C.; Gavagnin, M. Chemistry of two distinct Aeolid Spurilla species: ecological implications. Chem. Biodivers. 2017, 14, e1700125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Geng, H.-C.; Zhang, H.-B.; Dong, K.; Wang, W.-G.; Du, X.; Li, X.-N.; He, F.; Qin, H.-B.; Li, Y.; et al. Scopariusins, A new class of ent-halimane diterpenoids isolated from Isodon scoparius, and biomimetic synthesis of scopariusin A and isoscoparin N. Org. Lett. 2012, 15, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Lei, H. Diterpenoids of Gorgonian Corals: Chemistry and Bioactivity. Chem. Biodivers. 2016, 13, 345–365. [Google Scholar] [CrossRef]
- Walker, R.P.; Faulkner, D.J. Diterpenes from the sponge Dysidea amblia. J. Org. Chem. 1981, 46, 1098–1102. [Google Scholar] [CrossRef]
- Walker, R.P.; Rosser, R.M.; Faulkner, D.J.; Bass, L.S.; He, C.H.; Clardy, J. Two new metabolites of the sponge Dysidea amblia and revision of the structure of ambliol B. J. Org. Chem. 1984, 49, 5160–5163. [Google Scholar] [CrossRef]
- Shimbo, K.; Tsuda, M.; Fukushi, E.; Kawabata, J.; Kobayashi, J. Dytesinins A and B, new clerodane-type diterpenes with a cyclopropane ring from the tunicate Cystodytes sp. Tetrahedron 2000, 56, 7923–7926. [Google Scholar] [CrossRef]
- Gavagnin, M.; Trivellone, E.; Castelluccio, F.; Cimino, G.; Cattaneo-Vietti, R. Glyceryl ester of a new halimane diterpenoic acid from the skin of the antarctic nudibranch Austrodoris kerguelenensis. Tetrahedron Lett. 1995, 36, 7319–7322. [Google Scholar] [CrossRef]
- Maschek, J.A.; Mevers, E.; Diyabalanage, T.; Chen, L.; Ren, Y.; McClintock, J.B.; Amsler, C.D.; Wu, J.; Baker, B.J. Palmadorin chemodiversity from the antarctic nudibranch Austrodoris kerguelenensis and inhibition of Jak2/STAT5-dependent HEL leukemia cells. Tetrahedron 2012, 68, 9095–9104. [Google Scholar] [CrossRef]
- Soldatou, S.; Baker, B.J. Cold-water marine natural products, 2006 to 2016. Nat. Prod. Rep. 2017, 34, 585–626. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, M.; Chen, J.; Wang, H.; Tenney, K.; Crews, P. Bioactive secondary metabolites from the marine sponge genus Agelas. Mar. Drugs 2017, 15, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, K.; Criswell, J.; Zi, J.; Stubbs, A.; Peters, R.J. Novel product chemistry from mechanistic analysis of ent -copalyl diphosphate synthases from plant hormone biosynthesis. Angew. Chem. Int. Ed. 2014, 53, 7198–7202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcos, I.; Garcia, N.; Sexmero, M.; Basabe, P.; Díez, D.; Urones, J. Synthesis of (+)-agelasine C. A structural revision. Tetrahedron 2005, 61, 11672–11678. [Google Scholar] [CrossRef]
- Stout, E.P.; Yu, L.C.; Molinski, T.F. Antifungal diterpene alkaloids from the caribbean sponge Agelas citrina: unified configurational assignments of Agelasidines and Agelasines. Eur. J. Org. Chem. 2012, 2012, 5131–5135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, H.; Wu, H.; Ohizumi, Y.; Hirata, Y. Agelasine-A, -B, -C and -D, novel bicyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on Na,K-ATPase from the Okinawa sea sponge sp.1). Tetrahedron Lett. 1984, 25, 2989–2992. [Google Scholar] [CrossRef]
- Mancini, I.; Defant, A.; Guella, G. Recent synthesis of marine natural products with antibacterial activities. Anti-Infective Agents Med. Chem. 2007, 6, 17–48. [Google Scholar] [CrossRef]
- Hattori, T.; Adachi, K.; Shizuri, Y. New Agelasine compound from the marine sponge Agelas mauritiana as an antifouling substance against macroalgae. J. Nat. Prod. 1997, 60, 411–413. [Google Scholar] [CrossRef]
- Chu, M.-J.; Tang, X.-L.; Qin, G.-F.; Sun, Y.-T.; Li, L.; De Voogd, N.J.; Li, P.-L.; Li, G.-Q. Pyrrole derivatives and diterpene alkaloids from the south china sea sponge Agelas nakamurai. Chem. Biodivers. 2017, 14, e1600446. [Google Scholar] [CrossRef]
- Appenzeller, J.; Mihci, G.; Martin, M.-T.; Gallard, J.-F.; Menou, J.-L.; Boury-Esnault, N.; Hooper, J.; Petek, S.; Chevalley, S.; Valentin, A.; et al. Agelasines J, K, and L from the Solomon Islands marine sponge Agelas cf. mauritiana. J. Nat. Prod. 2008, 71, 1451–1454. [Google Scholar] [CrossRef]
- Kubota, T.; Iwai, T.; Takahashi-Nakaguchi, A.; Fromont, J.; Gonoi, T.; Kobayashi, J. Agelasines O–U, new diterpene alkaloids with a 9-N-methyladenine unit from a marine sponge Agelas sp. Tetrahedron 2012, 68, 9738–9744. [Google Scholar] [CrossRef]
- Ohba, M.; Iizuka, K.; Ishibashi, H.; Fujii, T. Syntheses and absolute configurations of the marine sponge purines (+)-agelasimine-A and (+)-agelasimine-B. Tetrahedron 1997, 53, 16977–16986. [Google Scholar] [CrossRef]
- Fathi-Afshar, R.; Allen, T.M. Biologically active metabolites from Agelas mauritiana. Can. J. Chem. 1988, 66, 45–50. [Google Scholar] [CrossRef]
- Peters, R.J. Two rings in them all: the labdane-related diterpenoids. Nat. Prod. Rep. 2010, 27, 1521–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.-C.; Feng, X.; Ko, T.-P.; Huang, C.-H.; Hu, Y.; Zheng, Y.; Bogue, S.; Nakano, C.; Hoshino, T.; Zhang, L.; et al. Structure and inhibition of tuberculosinol synthase and decaprenyl diphosphate synthase from Mycobacterium tuberculosis. J. Am. Chem. Soc. 2014, 136, 2892–2896. [Google Scholar] [CrossRef] [PubMed]
- Nakano, C.; Hoshino, T. Characterization of the Rv3377c gene product, a type-B diterpene cyclase, from the Mycobacterium tuberculosis H37 Genome. ChemBioChem 2009, 10, 2060–2071. [Google Scholar] [CrossRef]
- Jia, M.; Potter, K.; Peters, R.J. Extreme promiscuity of a bacterial and a plant diterpene synthase enables combinatorial biosynthesis. Metab. Eng. 2016, 37, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Adnani, N.; Braun, U.R.; Ellis, G.A.; Barns, K.J.; Parker-Nance, S.; Guzei, I.A.; Bugni, T.S. Micromonohalimanes A and B: Antibacterial halimane-type diterpenoids from a marine Micromonospora species. J. Nat. Prod. 2016, 79, 2968–2972. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, E. Tuberculosis terpene targets. Chem. Boil. 2015, 22, 437–438. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Ko, T.-P.; Chen, L.; Malwal, S.; Zhang, J.; Hu, X.; Qu, F.; Liu, W.; Huang, J.-W.; Cheng, Y.-S.; et al. “Head-to-middle” and “head-to-tail” cis-prenyl transferases: structure of isosesquilavandulyl diphosphate synthase. Angew. Chem. Int. Ed. 2018, 57, 683–687. [Google Scholar] [CrossRef]
- Silva, C.G.; Júnior, H.M.S.; Barbosa, J.P.; Costa, G.L.; Rodrigues, F.; De Oliveira, D.F.; Costa-Lotufo, L.V.; Alves, R.; Eleutherio, E.C.A.; De Rezende, C.M. Structure elucidation, antimicrobial and cytotoxic activities of a halimane isolated from Vellozia kolbekii ALVES (Velloziaceae). Chem. Biodivers. 2015, 12, 1891–1901. [Google Scholar] [CrossRef]
- Rijo, P.; Gaspar-Marques, C.; Simões, M.F.; Jimeno, M.L.; Rodríguez, B. Further diterpenoids from Plectranthus ornatus and P. grandidentatus. Biochem. Syst. Ecol. 2007, 35, 215–221. [Google Scholar] [CrossRef]
- Rijo, P.; Rodríguez, B.; Duarte, A.; Simões, M.F. Antimicrobial properties of Plectranthus ornatus extracts, 11-acetoxyhalima-5, 13-dien-15-oic acid metabolite and its derivatives. Nat. Prod. J. 2011, 1, 57–64. [Google Scholar]
- Burmistrova, O.; Simões, M.F.; Rijo, P.; Quintana, J.; Bermejo, J.; Estévez, F. Antiproliferative Activity of Abietane Diterpenoids against Human Tumor Cells. J. Nat. Prod. 2013, 76, 1413–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, K.; De Mieri, M.; Neuburger, M.; Zietsman, P.C.; Marston, A.; Van Vuuren, S.F.; Ferreira, D.; Hamburger, M.; Van Der Westhuizen, J.H. Labdane and clerodane diterpenoids from Colophospermum mopane. J. Nat. Prod. 2015, 78, 2494–2504. [Google Scholar] [CrossRef]
- Kihampa, C.; Nkunya, M.H.; Joseph, C.C.; Magesa, S.M.; Hassanali, A.; Heydenreich, M.; Kleinpeter, E. Anti-mosquito and antimicrobial nor-halimanoids, isocoumarins and an anilinoid from Tessmannia densiflora. Phytochemistry 2009, 70, 1233–1238. [Google Scholar] [CrossRef] [Green Version]
- Marcos, I.S.; Moro, R.F.; Costales, I.; Basabe, P.; Díez, D.; Mollinedo, F.; Urones, J.G. Synthesis of 12-epi-ent-polyalthenol an antitumour indole sesquiterpene alkaloid. Tetrahedron 2012, 68, 7932–7940. [Google Scholar] [CrossRef]
- Marcos, I.S.; Moro, R.F.; Costales, I.; Basabe, P.; Díez, D.; Mollinedo, F.; Urones, J.G. Biomimetic synthesis of an antitumour indole sesquiterpene alkaloid, 12-epi-ent-pentacyclindole. Tetrahedron 2013, 69, 7285–7289. [Google Scholar] [CrossRef]
- Marcos, I.S.; Moro, R.F.; Costales, I.; Escola, M.A.; Basabe, P.; Díez, D.; Urones, J.G. Synthesis of hexahydrocarbazoles by cyclisation of 3-(but-3-enyl) indole derivatives. Tetrahedron 2009, 65, 10235–10242. [Google Scholar] [CrossRef]
- Marcos, I.S.; Moro, R.F.; Costales, I.; Basabe, P.; Díez, D.; Gil, A.; Mollinedo, F.; La Rosa, F.P.-D.; Pérez-Roth, E.; Padrón, J.M. Synthesis and biological activity of polyalthenol and pentacyclindole analogues. Eur. J. Med. Chem. 2014, 73, 265–279. [Google Scholar] [CrossRef]
- García, P.A.; Valles, E.; Díez, D.; Castro, M.-Á. Marine alkylpurines: a promising group of bioactive marine natural products. Marine Drugs 2018, 16, 6. [Google Scholar] [CrossRef] [Green Version]
- Marcos, I.S.; Conde, A.; Moro, R.F.; Basabe, P.; Díez, D.; Urones, J.G. Synthesis of quinone/hydroquinone sesquiterpenes. Tetrahedron 2010, 66, 8280–8290. [Google Scholar] [CrossRef]
- Marcos, I.S.; Gonzalez, J.L.; Sexmero, M.J.; Díez, D.; Basabe, P.; Williams, D.J.; Simmonds, M.S.J.; Urones, J.G. Diterpenic α- and β-hydroxybutanolides with antifeedant activity: semisynthesis and absolute configuration. Tetrahedron Lett. 2000, 41, 2553–2557. [Google Scholar] [CrossRef]
- Ohba, M.; Kawase, N.; Fujii, T. Total syntheses of (±)-agelasimine-A, (±)-agelasimine-B, and (±)-purino-diterpene and the structure of diacetylagelasimine-A. J. Am. Chem. Soc. 1996, 118, 8250–8257. [Google Scholar] [CrossRef]
- Ohba, M.; Kawase, N.; Fujii, T.; Aoe, K.; Okamura, K.; Fathi-Afshar, R.; Allen, T.M. Racemic syntheses of agelasimine-A and agelasimine-B, bicyclic diterpenoids from the marine sponge Agelas mauritiana. Tetrahedron Lett. 1995, 36, 6101–6104. [Google Scholar] [CrossRef]
- Quilez del Moral, J.F.; Domingo, V.; Pérez, Á.; Martínez Andrade, K.A.; Enríquez, L.; Jaraiz, M.; López-Pérez, J.L.; Barrero, A.F. Mimicking halimane synthases: monitoring a cascade of cyclizations and rearrangements from epoxypolyprenes. J. Org. Chem. 2019, 84, 13764–13779. [Google Scholar] [CrossRef]
















| Marine Halimanes | Natural Sources | Activity | References |
|---|---|---|---|
| Nosyberkol, isotuberculosinol, 2 | Raspailia sp. M. tuberculosis | [29,32,33] | |
| Spurillin B, 8 | Spurilla sp. | [34] | |
| Echinohalimane A, 9 | Echinomuricea sp | Cytotoxic, neutrophil elastase inhibitor | [35,36] |
| Ambliol B, 10 | Dysidea amblia | [37,38] | |
| Ambliol C, 11 | D. amblia | [38] | |
| Dytesinin A, 12 | Cystodytes sp. | [39] | |
| Dytesinin B, 13 | Cystodytes sp. | [39] | |
| 14 | Echinomuricea sp. | Cytotoxic, anti-inflammatory | [39] |
| Marine Halimane-Glycerol Derivatives | Natural Sources | Activity | References |
|---|---|---|---|
| Austrodorin, 15 | Austrodoris kerguelenensis (Syn. of Doris kerguelenensis) | Self-defense | [40] |
| Diacetyl austrodorin, 16 | A. kerguelenensis | Self-defense | [40] |
| Palmadorin R, 17 | A. kerguelenensis | [41,42] | |
| Palmadorin S, 18 | A. kerguelenensis | [41,42] |
| Halimane-Purines | Natural Sources | Activity | References |
|---|---|---|---|
| (+)-8’-Oxo-agelasine C, 19 | Agelas mauritiana | [43] | |
| (−)-Agelasine C, 20 | Agelas sp. A. citrina | Inhibitory effects on Na,K-ATPase, antifungal, antimycobacterial | [45,46,47,48] |
| Epi-agelasine C, 21 | Agelas sp. | Antifouling, antimycobacterial | [45,48,49] |
| Isoagelasine C, 22 | A. nakamurai | Antifungal, antibacterial | [50] |
| (+)-Agelasine J, 23 | A. mauritiana | Antimalaria, antimicrobial, cytotoxic | [50,51] |
| (+)-Agelasine O, 24 | Agelas sp. | Antibacterial, antifungal | [52] |
| (+)-Agelasine S, 25 | Agelas sp. | Antibacterial, antifungal | [52] |
| (+)-Agelasimine A, 26 | A. mauritiana | Cytotoxic Adenosine transfer into rabbit erythrocytes inhibition. Ca2+-channel antagonistic action. α1 Adrenergic blockade | [53,54] |
| (+)-Agelasimine B, 27 | A. mauritiana | Cytotoxic Adenosine transfer into rabbit erythrocytes inhibition. Ca2+-channel antagonistic action. α1 Adrenergic blockade | [53,54] |
| Asmarine I, 28 | Raspailia sp. | Cytotoxic | [32] |
| Asmarine J, 29 | Raspailia sp. | Cytotoxic | [32] |
| Bacterial Halimanes | Natural Sources | Activity | References |
|---|---|---|---|
| Tuberculosinol, 1 | M. tuberculosis | [12,14,17,18,26,27,28,29,33] | |
| Nosyberkol, Isotuberculosinol, 2 | Raspailia sp. M. tuberculosis | [17,26,27,28,29,32,33] | |
| Tuberculosene, 30 | Kitasatospora griseola | [28,58] | |
| Micromonohalimane A, 31 | Micromonospora sp. | Antibacterial | [59] |
| Micromonohalimane B, 32 | Micromonospora sp. | Antibacterial | [59] |
| 1-Tuberculosinyl adenosine (1-TbAd), 3 | M. tuberculosis | M. tuberculosis biomarker | [19,20,60,61] |
| N6-Tuberculosinyl adenosine (N6-TbAd), 4 | M. tuberculosis | M. tuberculosis biomarker | [19,20,60] |
| Antibacterial Plant Halimanes | Natural Sources | Activity | References |
|---|---|---|---|
| 13R-ent-halim-1(10)-en-15,16-diol, 33 | Vellozia kolbekii | Antitumour, antimicrobial | [62] |
| 11R-Acetoxy-ent-halima-5,13E-dien-15-oic acid, 34 | Plectranthus ornatus | Antimicrobial | [63,64,65] |
| 35 | Colophospermum mopane | Antimicrobial | [66] |
| Tessmannic acid, 36 | Tessmannia densiflora | Antibacterial, antifungal, mosquito repellent, weak mosquitocidal | [67] |
| Tessmannic acid methyl ester, 37 | T. densiflora | Antibacterial, antifungal, mosquito repellent, weak mosquitocidal | [67] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobal, I.E.; Roncero, A.M.; Moro, R.F.; Díez, D.; Marcos, I.S. Antibacterial Natural Halimanes: Potential Source of Novel Antibiofilm Agents. Molecules 2020, 25, 1707. https://doi.org/10.3390/molecules25071707
Tobal IE, Roncero AM, Moro RF, Díez D, Marcos IS. Antibacterial Natural Halimanes: Potential Source of Novel Antibiofilm Agents. Molecules. 2020; 25(7):1707. https://doi.org/10.3390/molecules25071707
Chicago/Turabian StyleTobal, Ignacio E., Alejandro M. Roncero, Rosalina F. Moro, David Díez, and Isidro S. Marcos. 2020. "Antibacterial Natural Halimanes: Potential Source of Novel Antibiofilm Agents" Molecules 25, no. 7: 1707. https://doi.org/10.3390/molecules25071707
APA StyleTobal, I. E., Roncero, A. M., Moro, R. F., Díez, D., & Marcos, I. S. (2020). Antibacterial Natural Halimanes: Potential Source of Novel Antibiofilm Agents. Molecules, 25(7), 1707. https://doi.org/10.3390/molecules25071707