Aviculin Isolated from Lespedeza cuneata Induce Apoptosis in Breast Cancer Cells through Mitochondria-Mediated Caspase Activation Pathway
Abstract
:1. Introduction
2. Results
2.1. Effects of Four L. cuneata MeOH Extract Fractions (n-Hexane, CH2Cl2, EtOAc, and n-BuOH Fractions) on Metabolic Activity in MCF-7 Human Breast Cancer Cells
2.2. Isolation and Identification of Compounds from the EtOAc Fraction
2.3. Effects of Compounds 1–9 on Metabolic Activity in MCF-7 Human Breast Cancer Cells
2.4. Effects of Aviculin (2) on Nuclear Morphologies of MCF-7 Human Breast Cancer Cells
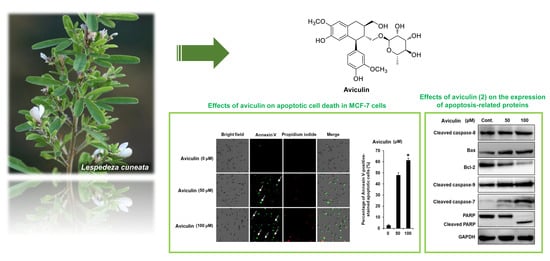
2.5. Effects of Aviculin (2) on Apoptotic Cell Death in MCF-7 Human Breast Cancer Cells
2.6. Effects of Aviculin (2) on the Expression of Apoptosis-related Proteins in MCF-7 Human Breast Cancer Cells
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Cell Culture
4.5. Ez-Cytox Assay
4.6. Western Blotting Analysis
4.7. Cell Staining with Annexin V
4.8. Cell Staining with Hoechst 33342
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, E.S.; Nho, J.H. Lifestyle Intervention for Breast Cancer Women. J. Lifestyle Med. 2019, 9, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, M.; Kostrzewska, B.; Kamińska, D.; Domurat, M.; Zińczuk, J.; Misiura, M.; Guzińska-Ustymowicz, K.; Pryczynicz, A. Symptoms of colorectal cancer contributes to its localization and advancement. Prog. Health Sci. 2019, 9, 76–82. [Google Scholar] [CrossRef]
- Neugut, A.I.; Hillyer, G.C.; Kushi, L.H.; Lamerato, L.; Nathanson, S.D.; Ambrosone, C.B.; Bovbjerg, D.H.; Mandelblatt, J.S.; Magai, C.; Tsai, W.Y. The Breast Cancer Quality of Care Study (BQUAL): A Multi-Center Study to Determine Causes for Noncompliance with Breast Cancer Adjuvant Therapy. Breast J. 2012, 18, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Sharma, B.R.; Rhyu, D.Y. Beneficial effect of Lespedeza cuneata (G. Don) water extract on streptozotocin-induced type 1 diabetes and cytokine-induced beta-cell damage. Nat. Prod. Sci. 2016, 22, 175–179. [Google Scholar] [CrossRef]
- Baek, J.; Lee, D.; Lee, T.K.; Song, J.H.; Lee, J.S.; Lee, S.; Yoo, S.W.; Kang, K.S.; Moon, E.; Lee, S.; et al. (−)-9′-O-(α-l-Rhamnopyranosyl) lyoniresinol from Lespedeza cuneata suppresses ovarian cancer cell proliferation through induction of apoptosis. Bioorg. Med. Chem. Lett. 2018, 28, 122–128. [Google Scholar] [CrossRef]
- Seong, J.S.; Xuan, S.H.; Park, S.H.; Lee, K.S.; Park, Y.M.; Park, S.N. Antioxidative and antiaging activities and component analysis of Lespedeza cuneata G. Don extracts fermented with Lactobacillus pentosus. J. Microbiol. Biotechnol. 2017, 27, 1961–1970. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Ji, J.; Park, S.H. Antiwrinkle and antimelanogenesis activity of the ethanol extracts of Lespedeza cuneata G. Don for development of the cosmeceutical ingredients. Food Sci. Nutr. 2018, 6, 1307–1316. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, C.W.; Kwon, J.E.; Negi, M.; Koo, Y.T.; Lee, S.H.; Baek, D.H.; Noh, Y.H.; Kang, S.C. Effects of Lespedeza cuneata aqueous extract on testosterone-induced prostatic hyperplasia. Pharm. Biol. 2019, 57, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Kwon, J.E.; Cho, S.M.; Kim, C.W.; Koo, Y.T.; Lee, S.H.; Lee, H.M.; Kang, S.C. Protective effect of Lespedeza cuneata ethanol extract on Bisphenol A-induced testicular dysfunction in vivo and in vitro. Biomed. Pharmacother. 2018, 102, 76–85. [Google Scholar] [CrossRef]
- Matsuura, S.; Iinuma, M.; Ito, E.; Takami, H.; Kagei, K. Studies on the constituents of the useful plants. VIII. The constituents of Lespedeza cuneata G. Don (author’s transl). Yakugaku Zasshi 1978, 98, 1542. [Google Scholar] [CrossRef] [Green Version]
- Yoo, G.; Park, S.J.; Lee, T.H.; Yang, H.; Baek, Y.S.; Kim, N.; Kim, Y.J.; Kim, S.H. Flavonoids isolated from Lespedeza cuneata G. Don and their inhibitory effects on nitric oxide production in lipopolysaccharide-stimulated BV-2 microglia cells. Pharmacogn. Mag. 2015, 11, 651. [Google Scholar] [PubMed] [Green Version]
- Zhang, C.F.; Zhou, J.; Yang, J.Z.; Li, C.J.; Ma, J.; Zhang, D.; Li, L.; Zhang, D.M. Three new lignanosides from the aerial parts of Lespedeza cuneata. J. Asian Nat. Prod. Res. 2016, 18, 913–920. [Google Scholar] [CrossRef] [PubMed]
- So, H.M.; Eom, H.J.; Lee, D.; Kim, S.; Kang, K.S.; Lee, I.K.; Baek, K.H.; Park, J.Y.; Kim, K.H. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharmacal Res. 2018, 41, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Roh, H.S.; Baek, K.H.; Lee, S.; Kim, S.; So, H.M.; Moon, E.; Pang, C.; Jang, T.S.; Kim, K.H. Bioactivity-guided isolation of ginsenosides from Korean Red Ginseng with cytotoxic activity against human lung adenocarcinoma cells. J. Ginseng Res. 2018, 42, 562–570. [Google Scholar] [CrossRef]
- Baek, S.C.; Choi, E.; Eom, H.J.; Jo, M.S.; Kim, S.; So, H.M.; Kim, S.H.; Kang, K.S.; Kim, K.H. LC/MS-based analysis of bioactive compounds from the bark of Betula platyphylla var. japonica and their effects on regulation of adipocyte and osteoblast differentiation. Nat. Prod. Sci. 2018, 24, 235–240. [Google Scholar]
- Lee, S.; Lee, S.; Roh, H.S.; Song, S.S.; Ryoo, R.; Pang, C.; Baek, K.H.; Kim, K.H. Cytotoxic constituents from the sclerotia of Poria cocos against human lung adenocarcinoma cells by inducing mitochondrial apoptosis. Cells 2018, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Trinh, T.A.; Park, E.J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.E. Estrogenic Activity of Sanguiin H-6 through Activation of Estrogen Receptor α Coactivator-binding Site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef]
- Baek, J.; Lee, T.; Song, J.H.; Choi, E.; Ko, H.J.; Lee, S.; Choi, S.; Lee, S.; Yoo, S.W.; Kim, S.H.; et al. Lignan Glycosides and Flavonoid Glycosides from the Aerial Portion of Lespedeza cuneata and Their Biological Evaluations. Molecules 2018, 23, 1920. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Li, C.J.; Yang, J.Z.; Ma, J.; Wu, L.Q.; Wang, W.J.; Zhang, D.M. Phenylpropanoid and lignan glycosides from the aerial parts of Lespedeza cuneata. Phytochemistry 2016, 121, 58–64. [Google Scholar] [CrossRef]
- Kaneda, N.; Kinghorn, A.D.; Farnsworth, N.R.; Tuchinda, P.; Udchachon, J.; Santisuki, T.; Reutrakul, V. Two diarylheptanoids and a lignan from Casuarina junghuhniana. Phytochemistry 1990, 29, 3366–3368. [Google Scholar] [CrossRef]
- Ochung, A.A.; Manguro, L.A.O.; Owuor, P.O.; Jondiko, I.O.; Nyunja, R.A.; Akala, H.; Mwinzi, P.; Opiyo, S.A. Bioactive carbazole alkaloids from Alysicarpus ovalifolius (Schumach). J. Korean Soc. Appl. Boil. Chem. 2015, 58, 839–846. [Google Scholar] [CrossRef]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Rayyan, S.; Fossen, T.; Nateland, H.S.; Anderson, O.M. Isolation and identification of flavonoids, including flavone rotamers, from the herbal drug ’Crataegi folium cum flore’ (hawthorn). Phytochem. Anal. 2005, 16, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.S.; Li, K.K.; Zheng, X.K. A new norlignan lignanoside from Selaginella moellendorffii Hieron. Acta Pharm. Sin. 2011, 1, 36–39. [Google Scholar] [CrossRef] [Green Version]
- Phadungkit, M.; Luanratana, O. Anti-Salmonella activity of constituents of Ardisia elliptica Thunb. Nat. Prod. Res. 2006, 20, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Ionkova, I. Anticancer lignans-from discovery to biotechnology. Mini-Rev. Med. Chem. 2011, 11, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Ionkova, I. Biotechnology and modern production of plant made pharmaceuticals: Anticancer compounds. Int. J. Curr. Chem. 2010, 1, 237–247. [Google Scholar]
- Imbert, T. Discovery of podophyllotoxins. Biochimie 1998, 80, 207–222. [Google Scholar] [CrossRef]
- Ohashi, K.; Winarno, H.; Mukai, M.; Inoue, M.; Prana, M.S.; Simanjuntak, P.; Shibuya, H. Indonesian medicinal plants. XXV. Cancer cell invasion inhibitory effects of chemical constituents in the parasitic plant Scurrula atropurpurea (Loranthaceae). Chem. Pharm. Bull. 2003, 51, 343–345. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Melino, G.; Knight, R.; Nicotera, P. How many ways to die? How many different models of cell death? Cell Death Differ. 2005, 12, 1457–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Herbert, P.; Warrens, A. An introduction to death receptors in apoptosis. Int. J. Surg. 2005, 3, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, C. (Ed.) Cell Death: The Role of PARP; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Soldani, C.; Scovassi, A. Poly (ADP-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis 2002, 7, 321–328. [Google Scholar] [CrossRef]
- Germain, M.; Affar, E.B.; D’Amours, D.; Dixit, V.M.; Salvesen, G.S.; Poirier, G.G. Cleavage of automodified poly (ADP-ribose) polymerase during apoptosis evidence for involvement of caspase-7. J. Biol. Chem. 1999, 274, 28379–28384. [Google Scholar] [CrossRef] [Green Version]
- Rossé, T.; Olivier, R.; Monney, L.; Rager, M.; Conus, S.; Fellay, I.; Jansen, B.; Borner, C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 1998, 391, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Jo, M.S.; Lee, D.; Baek, S.E.; Baek, J.; Yu, J.S.; Jo, J.; Yun, H.; Kang, K.S.; Yoo, J.E.; et al. Dual effects of isoflavonoids from Pueraria lobata roots on estrogenic activity and anti-proliferation of MCF-7 human breast carcinoma cells. Bioorg. Chem. 2019, 83, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, D.S.; Jung, K.; Hwang, G.S.; Lee, H.L.; Yamabe, N.; Lee, H.J.; Eom, D.W.; Kim, K.H.; Kang, K.S. Protective effect of ginsenoside Rb1 against tacrolimus-induced apoptosis in renal proximal tubular LLC-PK1 cells. J. Ginseng Res. 2018, 42, 75–80. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Lee, Y.H.; Lee, K.H.; Lee, B.S.; Alishir, A.; Ko, Y.-J.; Kang, K.S.; Kim, K.H. Aviculin Isolated from Lespedeza cuneata Induce Apoptosis in Breast Cancer Cells through Mitochondria-Mediated Caspase Activation Pathway. Molecules 2020, 25, 1708. https://doi.org/10.3390/molecules25071708
Lee D, Lee YH, Lee KH, Lee BS, Alishir A, Ko Y-J, Kang KS, Kim KH. Aviculin Isolated from Lespedeza cuneata Induce Apoptosis in Breast Cancer Cells through Mitochondria-Mediated Caspase Activation Pathway. Molecules. 2020; 25(7):1708. https://doi.org/10.3390/molecules25071708
Chicago/Turabian StyleLee, Dahae, Yong Hoon Lee, Kwang Ho Lee, Bum Soo Lee, Akida Alishir, Yoon-Joo Ko, Ki Sung Kang, and Ki Hyun Kim. 2020. "Aviculin Isolated from Lespedeza cuneata Induce Apoptosis in Breast Cancer Cells through Mitochondria-Mediated Caspase Activation Pathway" Molecules 25, no. 7: 1708. https://doi.org/10.3390/molecules25071708
APA StyleLee, D., Lee, Y. H., Lee, K. H., Lee, B. S., Alishir, A., Ko, Y.-J., Kang, K. S., & Kim, K. H. (2020). Aviculin Isolated from Lespedeza cuneata Induce Apoptosis in Breast Cancer Cells through Mitochondria-Mediated Caspase Activation Pathway. Molecules, 25(7), 1708. https://doi.org/10.3390/molecules25071708