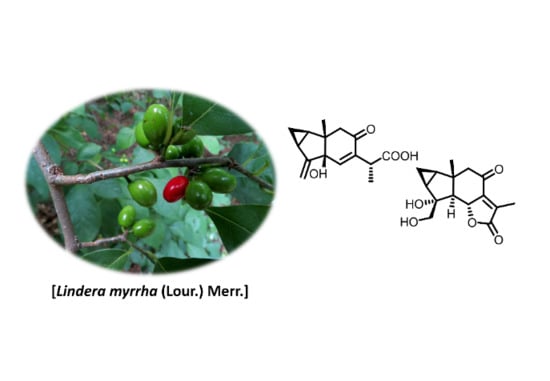
Atypical Lindenane-Type Sesquiterpenes from Lindera myrrha
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, M.-L.; Song, Y.; Ni, J.; Yao, X.; Tan, Y.-H.; Xu, Z.-F. Comparative chloroplast genomics and phylogenetics of nine Lindera species (Lauraceae). Sci. Rep. 2018, 8, 8844. [Google Scholar] [CrossRef]
- Cao, Y.; Xuan, B.; Peng, B.; Li, C.; Chai, X.; Tu, P. The genus Lindera: a source of structurally diverse molecules having pharmacological significance. Phytochem. Rev. 2016, 15, 869–906. [Google Scholar] [CrossRef]
- Tanaka, H.; Ichino, K.; Ito, K. A novel flavanone, linderatone, from Lindera umbellata. Chem. Pharm. Bull. (Tokyo) 1985, 33, 2602–2604. [Google Scholar] [CrossRef] [Green Version]
- Phan, B.-H.; Seguin, E.; Tillequin, F.; Koch, M. Aporphine alkaloids from Lindera myrrha. Phytochemistry 1994, 35, 1363–1365. [Google Scholar] [CrossRef]
- Duong, T.-H.; Nguyen, V.-K.; Nguyen-Pham, K.-T.; Sichaem, J.; Nguyen, H.-D. Lindermyrrhin, a novel 3,4-dihydroisocoumarin from Lindera myrrha roots. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Komae, H. Chemistry and distribution of sesquiterpene furans in Lauraceae. Biochem. Syst. Ecol. 1980, 8, 381–383. [Google Scholar] [CrossRef]
- Smith, S.G.; Goodman, J.M. Assigning Stereochemistry to Single Diastereoisomers by GIAO NMR Calculation: The DP4 Probability. J. Am. Chem. Soc. 2010, 132, 12946–12959. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; He, S.; Pan, Y. Sesquiterpenoid with new skeleton from Chloranthus henryi. Tetrahedron Lett. 2007, 48, 453–456. [Google Scholar] [CrossRef]
- Du, B.; Huang, Z.; Wang, X.; Chen, T.; Shen, G.; Fu, S.; Liu, B. A unified strategy toward total syntheses of lindenane sesquiterpenoid [4 + 2] dimers. Nat. Commun. 2019, 10, 1892. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Ichihara, M.; Okamoto, Y.; Gong, X.; Kuroda, C.; Tori, M. Twelve new compounds from Ligularia melanothyrsa; isolation of melanothyrsins A–E, normelanothyrsin A, and other eremophilane sesquiterpenoids. Tetrahedron 2014, 70, 2621–2628. [Google Scholar] [CrossRef]
- Shi, Y.-S.; Liu, Y.-B.; Ma, S.-G.; Li, Y.; Qu, J.; Li, L.; Yuan, S.-P.; Hou, Q.; Li, Y.-H.; Jiang, J.-D.; et al. Bioactive Sesquiterpenes and Lignans from the Fruits of Xanthium sibiricum. J. Nat. Prod. 2015, 78, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, H.; Hashimoto, A.; Fromont, J.; Hoshino, Y.; Mikami, Y.; Kobayashi, J. Halichonadins A–D, new sesquiterpenoids from a sponge Halichondria sp. Tetrahedron 2005, 61, 1101–1105. [Google Scholar] [CrossRef]
- Xu, Y.-J. Phytochemical and Biological Studies of Chloranthus Medicinal Plants. Chem. Biodivers. 2013, 10, 1754–1773. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, J.; Mizutani, J. Shizukanolides D, E and F, Novel Lindenanolides from Chloranthus spp. (Chloranthaceae). Agric. Biol. Chem. 1989, 53, 203–207. [Google Scholar] [CrossRef]
- Hu, X.; Yang, J.; Xu, X. Three Novel Sesquiterpene Glycosides of Sarcandra glabra. Chem. Pharm. Bull. (Tokyo) 2009, 57, 418–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, G.; Yang, L.; Yuan, C.; Du, B.; Liu, B. Total syntheses of lindenane-type sesquiterpenoids: (±)-chloranthalactones A, B, F, (±)-9-hydroxy heterogorgiolide, and (±)-shizukanolide E. Tetrahedron 2012, 68, 9624–9637. [Google Scholar] [CrossRef]
- Fenlon, T.W.; Jones, M.W.; Adlington, R.M.; Lee, V. Synthesis of rac-Lindenene via a thermally induced cyclopropanation reaction. Org. Biomol. Chem. 2013, 11, 8026–8029. [Google Scholar] [CrossRef]
- Wu, B.; Chen, J.; Qu, H.; Cheng, Y. Complex Sesquiterpenoids with Tyrosinase Inhibitory Activity from the Leaves of Chloranthus tianmushanensis. J. Nat. Prod. 2008, 71, 877–880. [Google Scholar] [CrossRef]
- Cao, C.-M.; Peng, Y.; Shi, Q.-W.; Xiao, P.-G. Chemical Constituents and Bioactivities of Plants of Chloranthaceae. Chem. Biodivers. 2008, 5, 219–238. [Google Scholar] [CrossRef]
- Wang, A.-R.; Song, H.-C.; An, H.-M.; Huang, Q.; Luo, X.; Dong, J.-Y. Secondary Metabolites of Plants from the Genus Chloranthus: Chemistry and Biological Activities. Chem. Biodivers. 2015, 12, 451–473. [Google Scholar] [CrossRef] [PubMed]
- He, X.-F.; Yin, S.; Ji, Y.-C.; Su, Z.-S.; Geng, M.-Y.; Yue, J.-M. Sesquiterpenes and Dimeric Sesquiterpenoids from Sarcandra glabra. J. Nat. Prod. 2010, 73, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Zhang, H.; Liu, H.-C.; Yang, S.-P.; Geng, M.-Y.; Yue, J.-M. Cytotoxic sesquiterpenoids from Sarcandra glabra. Tetrahedron 2013, 69, 564–569. [Google Scholar] [CrossRef]
- Kouno, I.; Hirai, A.; Fukushige, A.; Jiang, Z.-H.; Tanaka, T. New Eudesmane Sesquiterpenes from the Root of Lindera strychnifolia. J. Nat. Prod. 2001, 64, 286–288. [Google Scholar] [CrossRef]
- Zhang, C.; Nakamura, N.; Tewtrakul, S.; Hattori, M.; Sun, Q.; Wang, Z.; Fujiwara, T. Sesquiterpenes and Alkaloids from Lindera chunii and Their Inhibitory Activities against HIV-1 Integrase. Chem. Pharm. Bull. 2002, 50, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, L.-S.; Zheng, Y.-L.; Mo, J.-X.; Liu, X.; Li, X.-H.; Zhou, C.-X. Sesquiterpene Lactones from the Root Tubers of Lindera aggregata. J. Nat. Prod. 2009, 72, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Xiong, J.; Liu, S.-T.; Liu, X.-H.; Hu, J.-F. Sesquiterpenoids from Chloranthus henryi and Their Anti-neuroinflammatory Activities. Chem. Biodivers. 2014, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, J.; Fukushi, Y.; Tahara, S.; Mizutani, J. Shizukaol a, a sesquiterpene dimer from Chloranthus japonicus. Phytochemistry 1990, 29, 2332–2334. [Google Scholar] [CrossRef]
- Kawabata, J.; Mizutani, J. Dimeric sesquiterpenoid esters from Chloranthus serratus. Phytochemistry 1992, 31, 1293–1296. [Google Scholar] [CrossRef]
- Kawabata, J.; Fukushi, E.; Mizutani, J. Sesquiterpene dimers from Chloranthus japonicus. Phytochemistry 1995, 39, 121–125. [Google Scholar] [CrossRef]
- Yang, S.-P.; Gao, Z.-B.; Wang, F.-D.; Liao, S.-G.; Chen, H.-D.; Zhang, C.-R.; Hu, G.-Y.; Yue, J.-M. Chlorahololides A and B, Two Potent and Selective Blockers of the Potassium Channel Isolated from Chloranthus holostegius. Org. Lett. 2007, 9, 903–906. [Google Scholar] [CrossRef]
- Yang, S.-P.; Gao, Z.-B.; Wu, Y.; Hu, G.-Y.; Yue, J.-M. Chlorahololides C–F: a new class of potent and selective potassium channel blockers from Chloranthus holostegius. Tetrahedron 2008, 64, 2027–2034. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kashiwada, Y.; Kawazoe, K.; Murakami, K.; Sun, H.-D.; Li, S.-L.; Takaishi, Y. Spicachlorantins C–F, hydroperoxy dimeric sesquiterpenes from the roots of Chloranthus spicatus. Tetrahedron Lett. 2009, 50, 6032–6035. [Google Scholar] [CrossRef]
- Yan, H.; Ba, M.-Y.; Li, X.-H.; Guo, J.-M.; Qin, X.-J.; He, L.; Zhang, Z.-Q.; Guo, Y.; Liu, H.-Y. Lindenane sesquiterpenoid dimers from Chloranthus japonicus inhibit HIV-1 and HCV replication. Fitoterapia 2016, 115, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Du, B.; Deng, H.; Man, Y.; Liu, B. Total Syntheses of Sarcandrolide J and Shizukaol D: Lindenane Sesquiterpenoid [4+2] Dimers. Angew. Chem. Int. Ed. 2017, 56, 637–640. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09 Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Konstantinov, I.A.; Broadbelt, L.J. Regression formulas for density functional theory calculated 1H and 13C NMR chemical shifts in toluene-d8. J. Phys. Chem. A 2011, 115, 12364–12372. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism: I. A gauge-invariant LCAO method for NMR chemical shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 1 and 2 are not available from the authors. |




| 1 | 2 | |||
|---|---|---|---|---|
| δC | δH (J, Hz) | δC | δH (J, Hz) | |
| 1 | 31.1 | 1.46, 1H, m | 29.1 | 1.49, 1H, m |
| 2 | 13.3 | 1.52, 1H, m | 12.3 | 1.36, 1H, m |
| 0.71, 1H, m | 0.70, 1H, m | |||
| 3 | 23.4 | 2.00, 1H, m | 28.7 | 1.87, 1H, m |
| 4 | 155.9 | - | 80.1 | - |
| 5 | 76.5 | - | 63.8 | 2.27, 1H, d, 12.0 |
| 6 | 145 | 6.65, 1H, s | 78.4 | 5.03, 1H, dq, 12.0, 2.0 |
| 7 | 136.1 | - | 154.6 | - |
| 8 | 197.7 | - | 197.7 | - |
| 8 | 150 | - | 148.7 | - |
| 9 | 51.2 | 2.39, 1H, d, 15.5 | 56.5 | 2.67, 1H, d, 16.0 |
| 2.31, 1H, d, 15.5 | 2.62, 1H, d, 16.0 | |||
| 10 | 50.8 | - | 41.4 | - |
| 11 | 38.9 | 3.50, 1H, q, 7.0 | 132.1 | - |
| 12 | 174 | - | 173.9 | - |
| 13 | 16.6 | 1.26, 3H, d, 7.0 | 9.8 | 1.92, 3H, s |
| 14 | 18.3 | 1.13, 3H, s | 21.9 | 1.11, 3H, s |
| 15 | 109 | 5.17, 1H, s | 68.2 | 3.67, 1H, d, 10.5 |
| 4.99, 1H, s | 3.80, 1H, d, 10.5 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, T.-H.; Beniddir, M.A.; Trung, N.T.; Phan, C.-T.D.; Vo, V.G.; Nguyen, V.-K.; Le, Q.-L.; Nguyen, H.-D.; Le Pogam, P. Atypical Lindenane-Type Sesquiterpenes from Lindera myrrha. Molecules 2020, 25, 1830. https://doi.org/10.3390/molecules25081830
Duong T-H, Beniddir MA, Trung NT, Phan C-TD, Vo VG, Nguyen V-K, Le Q-L, Nguyen H-D, Le Pogam P. Atypical Lindenane-Type Sesquiterpenes from Lindera myrrha. Molecules. 2020; 25(8):1830. https://doi.org/10.3390/molecules25081830
Chicago/Turabian StyleDuong, Thuc-Huy, Mehdi A. Beniddir, Nguyen T. Trung, Cam-Tu D. Phan, Van Giau Vo, Van-Kieu Nguyen, Quynh-Loan Le, Hoang-Dung Nguyen, and Pierre Le Pogam. 2020. "Atypical Lindenane-Type Sesquiterpenes from Lindera myrrha" Molecules 25, no. 8: 1830. https://doi.org/10.3390/molecules25081830
APA StyleDuong, T. -H., Beniddir, M. A., Trung, N. T., Phan, C. -T. D., Vo, V. G., Nguyen, V. -K., Le, Q. -L., Nguyen, H. -D., & Le Pogam, P. (2020). Atypical Lindenane-Type Sesquiterpenes from Lindera myrrha. Molecules, 25(8), 1830. https://doi.org/10.3390/molecules25081830