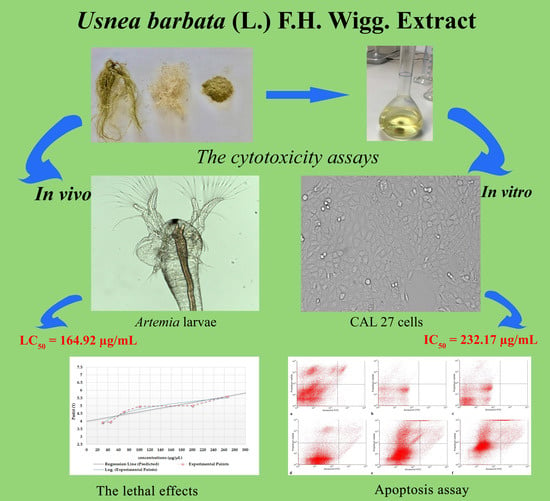
Evaluation of the Cytotoxic Activity of the Usnea barbata (L.) F. H. Wigg Dry Extract
Abstract
:1. Introduction
2. Results
2.1. Determination of Usnic Acid Content in Different Extracts of Usnea barbata (L.) F. H. Wigg
2.2. Evaluation of UBE Cytotoxic Activity by BSL Assay
2.3. Effects of UBE Treatment on Morphological Characteristics of CAL 27 (ATCC® CRL-2095™) Cell Line
2.4. In Vitro Cytotoxicity of UBE on CAL 27 (ATCC® CRL-2095™) Cell Line
2.4.1. MTT Assay
2.4.2. Apoptosis Assay
2.4.3. Assessment of Antioxidant Enzyme Activity
3. Discussions
4. Materials and Methods
4.1. Lichen Samples
4.2. Preparation of Lichen Extracts
4.3. Determination of Usnic Acid Content in Different Extracts of Usnea barbata (L.) F. H. Wigg.
4.3.1. HPLC Analysis of Usnic Acid in Various Liquid Extracts of Usnea barbata (L.) F. H. Wigg
4.3.2. UHPLC Analysis of Usnic Acid in UBE
4.4. Evaluation of UBE Cytotoxic Activity by BSL Assay
4.5. Effects of UBE Treatment on Morphological Characteristics of CAL 27 (ATCC® CRL-2095™) Cell Line
4.6. In Vitro Cytotoxicity of UBE on CAL 27 (ATCC® CRL-2095™) Cell Line
4.6.1. MTT Assay
4.6.2. Apoptosis Assay
4.6.3. Assessment of Antioxidant Enzyme Activity
Determination of Superoxide Dismutase Activity
Determination of Catalase Activity
Determination of Peroxidase Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Klaunig, J.E.; Wang, Z. Oxidative stress in carcinogenesis. Curr. Opin. Toxicol. 2018, 7, 116–121. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. Ros homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Chen, H.C.; Wan, X.X.; Tania, M.; Xu, A.H.; Chen, F.Z.; Zhang, D.Z. Regulatory effects of resveratrol on antioxidant enzymes: A mechanism of growth inhibition and apoptosis induction in cancer cells. Mol. Cells 2013, 35, 219–225. [Google Scholar] [CrossRef]
- Di Meo, S.; Napolitano, G.; Venditti, P. Physiological and pathological role of ROS: Benefits and limitations of antioxidant treatment. Int. J. Mol. Sci. 2019, 20, 4810. [Google Scholar] [CrossRef] [Green Version]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef]
- Huber, M.A.; Tantiwongkosi, B. Oral and Oropharyngeal Cancer. Med. Clin. N. Am. 2014, 98, 1299–1321. [Google Scholar] [CrossRef]
- Ettinger, K.S.; Ganry, L.; Fernandes, R.P. Oral Cavity Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 13–29. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, G.; St Clair, L.L. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- Richardson, D.H.S. War in the world of lichens: Parasitism and symbiosis as exemplified by lichens and lichenicolous fungi. Mycol. Res. 1999, 106, 641–650. [Google Scholar] [CrossRef]
- Ranković, B. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential, 2nd ed.; Springer Nature Switzerland AG: Basel, Switzerland, 2019; ISBN 978-3-030-16813-1. [Google Scholar]
- Yeash, E.A.; Letwin, L.; Malek, L.; Suntres, Z.; Knudsen, K.; Christopher, L.P. Biological activities of undescribed North American lichen species. J. Sci. Food Agric. 2017, 97, 4721–4726. [Google Scholar] [CrossRef]
- Shcherbokova, A.; Koptina, A.; Samiento-Diaz, M.; Ulrich-Merzenich, G. Pharmacological activities of lichens from Russia. Planta Med. 2015, 81, 1462. [Google Scholar] [CrossRef]
- Prateeksha, G.; Paliya, B.S.; Bajpai, R.; Jadaun, V.; Kumar, J.; Kumar, S.; Upreti, D.K.; Singh, B.R.; Nayaka, S.; Joshi, Y.; et al. The genus Usnea: A potent phytomedicine with multifarious ethnobotany, phytochemistry and pharmacology. RSC Adv. 2016, 6, 21672–21696. [Google Scholar] [CrossRef]
- Bazarnova, Y.; Politaeva, N.; Lyskova, N. Research for the lichen Usnea barbata metabolites. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2018, 73, 291–296. [Google Scholar] [CrossRef]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary metabolite profiling of species of the genus Usnea by UHPLC-ESI-OT-MS-MS. Molecules 2017, 23, 54. [Google Scholar] [CrossRef] [Green Version]
- Divya Reddy, S.; Siva, B.; Kumar, K.; Phani Babu, V.S.; Sravanthi, V.; Boustie, J.; Lakshma Nayak, V.; Tiwari, A.K.; Rao, C.H.V.; Sridhar, B.; et al. Comprehensive Analysis of Secondary Metabolites in Usnea longissima (Lichenized Ascomycetes, Parmeliaceae) Using UPLC-ESI-QTOF-MS/MS and Pro-Apoptotic Activity of Barbatic Acid. Molecules 2019, 24, 2270. [Google Scholar] [CrossRef] [Green Version]
- Cocchietto, M.; Skert, N.; Nimis, P.; Sava, G. A review on usnic acid, an interesting natural compound. Naturwissenschaften 2002, 89, 137–146. [Google Scholar] [CrossRef]
- Güran, Ş.; Çoban, Z.D.; Karaer, T.; Atmaca, B.; Kaya Demir, H. Usnic acid uses mitochondrial apopitotic pathway in it’s antitumoral role. Cumhur. Med. J. 2017, 39, 539–545. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M.; Stanojković, T.; Vasiljević, P.; Manojlović, N. Biological Activities of Toninia candida and Usnea barbata Together with Their Norstictic Acid and Usnic Acid Constituents. Int. J. Mol. Sci. 2012, 13, 14707–14722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Determination of the content in usnic acid and polyphenols from the extracts of Usnea barbata L. and the evaluation of their antioxidant activity. Farmacia 2018, 66, 337–341. [Google Scholar]
- Popovici, V.; Bucur, L.; Costache, T.; Gherghel, D.; Vochita, G.; Mihai, C.T.; Rotinberg, P.; Schroder, V.; Badea, F.C.; Badea, V. Studies on Preparation and UHPLC Analysis of the Usnea barbata (L) F.H.Wigg Dry acetone extract. Rev. Chim. 2019, 70, 3775–3777. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Q.; Fang, J.L.; Mei, N.; Ali, A.A.; Lewis, S.M.; Leakey, J.E.A.; Frankos, V.H. Review of usnic acid and Usnea barbata toxicity. J. Environ. Sci. Health - Part C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Cansaran, D.; Kahya, D.; Yurdakulol, E.; Atakol, O. Identification and quantitation of usnic acid from the lichen Usnea species of Anatolia and antimicrobial activity. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2006, 61, 773–776. [Google Scholar] [CrossRef]
- Zugic, A.; Jeremic, I.; Isakovic, A.; Arsic, I.; Savic, S.; Tadic, V. Evaluation of anticancer and antioxidant activity of a commercially available CO2 supercritical extract of old man’s beard (Usnea barbata). PLoS ONE 2016, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kristmundsdóttir, T.; Jónsdóttir, E.; Ögmundsdóttir, H.M.; Ingólfsdóttir, K. Solubilization of poorly soluble lichen metabolites for biological testing on cell lines. Eur. J. Pharm. Sci. 2005, 24, 539–543. [Google Scholar] [CrossRef]
- Fanum, M. Colloids in Drug Delivery; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4398-1825-1. [Google Scholar]
- Zaini, E.; Nisak, R.K.; Utami, R.D.; Fitriani, L.; Ismed, F. Effect of milling on physicochemical properties of usnic acid isolated from Usnea sp. Orient. J. Chem. 2017, 33. [Google Scholar] [CrossRef]
- Coiffard, C.; Coiffard, L.; De Roeck-Holtzhauer, Y. Comparison of thermostability of natural (sulfoprolamine and sodium usnate) and synthetic (climbazol and piroctone olamine) antidandruff agents. Ann. Pharm. Fr. 1999, 57, 392–396. [Google Scholar] [PubMed]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.; Bhattarai, H.D.; Pandey, D.P.; Hur, J.S.; Hong, S.G.; Kim, I.-C.; Yim, J.H. Antioxidant, Antibacterial activity and Brine shrimp toxicity test of some Mountainous Lichens from Nepal. Biol. Res. 2012, 45, 387–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harwig, J.; Scott, P. Brine shrimp (Artemia salina L.) larvae as a screening system for fungal toxins. Appl. Microbiol. 1971, 21, 1011–1016. [Google Scholar] [CrossRef] [Green Version]
- Kerster, H.W.; Schaeffer, D.J. Brine shrimp (Artemia salina) nauplii as a teratogen test system. Ecotoxicol. Environ. Safe 1983, 7, 342–349. [Google Scholar] [CrossRef]
- Ravaglia, L.M.; Gonçalves, K.; Oyama, N.M.; Coelho, R.G.; Spielmann, A.A.; Honda, N.K. In vitro radical-scavenging activity, toxicity against A. salina, and nmr profiles of extracts of lichens collected from Brazil and Antarctica. Quim. Nova 2014, 37, 1015–1021. [Google Scholar] [CrossRef]
- Sundquist, T.; Moravec, R.; Niles, A.; O’Brien, M.; Riss, T. Timing your apoptosis assays. Cell Notes 2006. [Google Scholar]
- Rabelo, T.K.; Zeidán-Chuliá, F.; Vasques, L.M.; dos Santos, J.P.A.; da Rocha, R.F.; de Bittencourt Pasquali, M.A.; Rybarczyk-Filho, J.L.; Araújo, A.A.S.; Moreira, J.C.F.; Gelain, D.P. Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicol. Vitr. 2012, 26, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Ben Mrid, R.; Bouchmaa, N.; Bouargalne, Y.; Ramdan, B.; Karrouchi, K.; Kabach, I.; El Karbane, M.; Idir, A.; Zyad, A.; Nhiri, M. Phytochemical Characterization, Antioxidant and In Vitro Cytotoxic Activity Evaluation of Juniperus oxycedrus Subsp. oxycedrus Needles and Berries. Molecules 2019, 24, 502. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Tania, M.; Zhang, D.Z.; Chen, H.C. Antioxidant enzymes and cancer. Chin. J. Cancer Res. 2010, 22, 87–92. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laville, N.; Aït-Ässa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assay—MTT. In Methods in Molecular Biology; Springer Nature Switzerland AG: Basel, Switzerland, 2011. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Telford, W.; Skommer, J.; Darzynkiewicz, Z. Apoptosis and Beyond: Cytometry in Studies of Programmed Cell Death. Methods Cell Biol. 2011, 103, 55–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified Annexin V/Propidium Iodide Apoptosis Assay for Accurate Assessment of Cell Death. J. Vis. Exp. 2011, 50, e2597. [Google Scholar] [CrossRef]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Martins, V.C.; Prazeres, D.M.F.; Vojinović, V.; Cabral, J.M.S.; Fonseca, L.P. Horseradish peroxidase: A valuable Tool in Biotechnology. Biotechnol. Annu. Rev. 2003, 9, 199–247. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, O. Maths from Scratch for Biologists. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 390. [Google Scholar] [CrossRef]
Sample Availability: Not available. |










| Usnea barbata (L.) Extract | Usnic Acid Content (g %) | Method |
|---|---|---|
| Aqueous extract | 0.04 | HPLC |
| Ethanol macerate | 0.26 | HPLC |
| Acetone macerate | 2.12 | HPLC |
| Dry acetone extract in DMSO | 16.53 | UHPLC |
| Dry acetone extract in acetone | 31.59 | UHPLC |
| Contact Solution | U SOD/g Protein | U CAT/g Protein | U POD/g Protein |
|---|---|---|---|
| Control | 3.111 ± 0.511 | 0.944 ± 0.274 | 0.0053 ± 0.0002 |
| UBE 100 µg/mL | 4.429 ± 0.550 | 1.499 ± 0.287 | 0.0154 ± 0.0005 |
| UBE 400 µg/mL | 4.599 ± 0.627 | 3.735 ± 0.418 | 0.0170 ± 0.0014 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, V.; Bucur, L.A.; Schröder, V.; Gherghel, D.; Mihai, C.T.; Caraiane, A.; Badea, F.C.; Vochița, G.; Badea, V. Evaluation of the Cytotoxic Activity of the Usnea barbata (L.) F. H. Wigg Dry Extract. Molecules 2020, 25, 1865. https://doi.org/10.3390/molecules25081865
Popovici V, Bucur LA, Schröder V, Gherghel D, Mihai CT, Caraiane A, Badea FC, Vochița G, Badea V. Evaluation of the Cytotoxic Activity of the Usnea barbata (L.) F. H. Wigg Dry Extract. Molecules. 2020; 25(8):1865. https://doi.org/10.3390/molecules25081865
Chicago/Turabian StylePopovici, Violeta, Laura Adriana Bucur, Verginica Schröder, Daniela Gherghel, Cosmin Teodor Mihai, Aureliana Caraiane, Florin Ciprian Badea, Gabriela Vochița, and Victoria Badea. 2020. "Evaluation of the Cytotoxic Activity of the Usnea barbata (L.) F. H. Wigg Dry Extract" Molecules 25, no. 8: 1865. https://doi.org/10.3390/molecules25081865
APA StylePopovici, V., Bucur, L. A., Schröder, V., Gherghel, D., Mihai, C. T., Caraiane, A., Badea, F. C., Vochița, G., & Badea, V. (2020). Evaluation of the Cytotoxic Activity of the Usnea barbata (L.) F. H. Wigg Dry Extract. Molecules, 25(8), 1865. https://doi.org/10.3390/molecules25081865