Bioassay-Guided Isolation of Antiproliferative Compounds from Limbarda crithmoides (L.) Dumort
Abstract
:1. Introduction
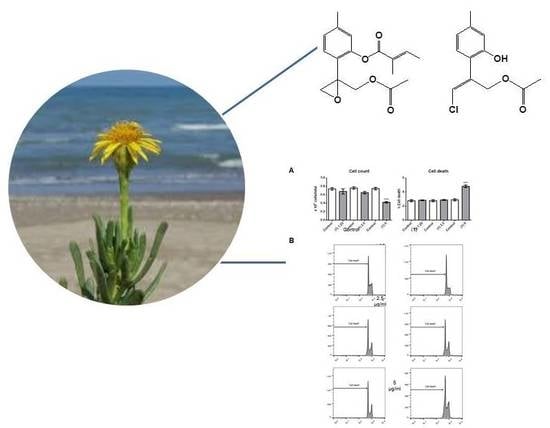
2. Results and Discussion
3. Materials and Methods
3.1. General Chemical Material
3.2. Plant Material
3.3. Extraction and Isolation Procedure
3.4. Cell Line Culture and Characterisation
3.5. Analysis of Cell Viability and Cell Cycle Progression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Plant List: A Working List of All Plant Species. Available online: http://www.theplantlist.org (accessed on 10 October 2019).
- Zurayk, R.A.; Baalbaki, R. Inula crithmoides: A candidate plant for saline agriculture. Arid Soil Res. Rehabil. 1996, 10, 213–223. [Google Scholar] [CrossRef]
- Tardio, J.; Pardo de Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Salerno, G.; Caneva, G. Food, flavouring and feed plant traditions in the Tyrrhenian sector of Basilicata, Italy. J. Ethnobiol. Ethnomed. 2006, 2, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Asteraceae species with most prominent bioactivity and their potential applications: A review. Ind. Crop. Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Harispe-Grandperrin, M.; Harispe, J.V. Proximate principles of Inula crithmoides L. family Compositae. II. Extraction and physical constants of the essential oil from the aerial parts. Bull. Soc. Chim. Biol. 1943, 25, 418–421. [Google Scholar]
- Harispe-Grandperrin, M.; Harispe, J.-V. Proximate principles of Inula crithmoides. III. Preliminary examination of the essential oil of the flower heads. Bull. Soc. Chim. Biol. 1944, 26, 192–202. [Google Scholar]
- Tsoukatou, M.; Roussis, V. Chemical composition and intra Mediterranean variation of the Inula crithmoides m L. oil. J. Essent. Oil Res. 1999, 11, 199–202. [Google Scholar] [CrossRef]
- Omezzine, F.; Daami-Remadi, M.; Rinez, A.; Ladhari, A.; Haouala, R. In vitro assessment of Inula spp. organic extracts for their antifungal activity against some pathogenic and antagonistic fungi. Afr. J. Microbiol. Res. 2011, 5, 3527–3531. [Google Scholar]
- Bucchini, A.; Giamperi, L.; Ricci, D. Total polyphenol content, in vitro antifungal and antioxidant activities of callus cultures from Inula crithmoides L. Nat. Prod. Commun. 2013, 8, 1587–1590. [Google Scholar] [CrossRef] [Green Version]
- Bucchini, A.; Ricci, D.; Messina, F.; Marcotullio, M.C.; Curini, M.; Giamperi, L. Antioxidant and antifungal activity of different extracts obtained from aerial parts of Inula crithmoides L. Nat. Prod. Res. 2015, 29, 1173–1176. [Google Scholar] [CrossRef]
- Jallali, I.; Waffo Teguo, P.; Smaoui, A.; Merillon, J.-M.; Abdelly, C.; Ksouri, R. Bio-guided fractionation and characterization of powerful antioxidant compounds from the halophyte Inula crithmoides. Arab. J. Chem. 2020, 13, 2680–2688. [Google Scholar] [CrossRef]
- Andreani, S.; De Cian, M.-C.; Paolini, J.; Desjobert, J.-M.; Costa, J.; Muselli, A. Chemical variability and antioxidant activity of Limbarda crithmoides L. essential oil from Corsica. Chem. Biodivers. 2013, 10, 2061–2077. [Google Scholar] [CrossRef] [PubMed]
- Giamperi, L.; Bucchini, A.; Fraternale, D.; Genovese, S.; Curini, M.; Ricci, D. Composition and antioxidant activity of Inula crithmoides essential oil grown in central Italy (Marche region). Nat. Prod. Commun. 2010, 5, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoides L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef]
- Aboul Ela, M.A.; El-Lakany, A.M.; Abdel-Kader, M.S.; Alqasoumi, S.I.; Shams-El-Din, S.M.; Hammoda, H.M. New quinic acid derivatives from hepatoprotective Inula crithmoides root extract. Helv. Chim. Acta 2012, 95, 61–66. [Google Scholar]
- Males, Z.; Plazibat, M.; Greiner, M. Qualitative and quantitative analysis of flavonoids of golden samphire -Limbarda crithmoides (L.) Dumort. Farm. Glas. 2004, 60, 453–459. [Google Scholar]
- Malash, B.N.; Ibrahim, S.M.; Ibrahim, A.-R.S.; Kabbash, A.; El-Aasr, M. In vitro and in vivo hepatoprotective study of Inula crithmoides L. Pluchea dioscoridis (L.) Desf. and Phyllanthus reticulates Poir. J. Pharm. Sci. Res. 2015, 7, 987–993. [Google Scholar]
- Assi, M.; Aboul-Ela, M.A.; Ellakany, A.M.; Abdul-Ghani, M. A comparative phytochemical and antimicrobial analysis of Inula viscosa and Inula crithmoides grown in Lebanon. Acad. J. Med. Plants 2014, 2, 057–067, 11 pp. [Google Scholar]
- Aboul Ela, M.A.; El-Lakany, A.M.; Shams-El-Din, S.M.; Hammoda, H.M. Phytochemical and antimicrobial investigation of Inula crithmoides L. Alexandria J. Pharm. Sci. 2011, 25, 37–40. [Google Scholar]
- Oliveira, M.; Joao Rodrigues, M.; Pereira, C.; Neto, R.L.D.M.; Junior, P.A.S.; Neng, N.D.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custodio, L. First report of the in vitro antileishmanial properties of extremophile plants from the Algarve Coast. Nat. Prod. Res. 2018, 32, 600–604. [Google Scholar] [CrossRef]
- Adorisio, S.; Fierabracci, A.; Gigliarelli, G.; Muscari, I.; Cannarile, L.; Liberati, A.M.; Marcotullio, M.C.; Riccardi, C.; Curini, M.; Robles Zepeda, R.E.; et al. The hexane fraction of Bursera microphylla A Gray induces p21-mediated antiproliferative and proapoptotic effects in human cancer-derived cell lines. Integr. Cancer Ther. 2017, 16, 426–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gigliarelli, G.; Zadra, C.; Cossignani, L.; Robles Zepeda, R.E.; Rascon-Valenzuela, L.A.; Velazquez-Contreras, C.A.; Marcotullio, M.C. Two new lignans from the resin of Bursera microphylla A. Gray and their cytotoxic activity. Nat. Prod. Res. 2018, 32, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Marcotullio, M.C.; Loizzo, M.R.; Messina, F.; Temperini, A.; Tundis, R.; Menichini, F.; Curini, M. Bioassay-guided fractionation of Euphrasia pectinata Ten. and isolation of iridoids with antiproliferative activity. Phytochem. Lett. 2015, 12, 252–256. [Google Scholar] [CrossRef]
- Messina, F.; Curini, M.; Di Sano, C.; Zadra, C.; Gigliarelli, G.; Rascon-Valenzuela, L.A.; Robles Zepeda, R.E.; Marcotullio, M.C. Diterpenoids and triterpenoids from the Resin of Bursera microphylla and their cytotoxic activity. J. Nat. Prod. 2015, 78, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Torres-Moreno, H.; Velazquez, C.A.; Garibay-Escobar, A.; Curini, M.; Marcotullio, M.C.; Robles-Zepeda, R.E. Antiproliferative and apoptosis induction of cucurbitacin-type triterpenes from Ibervillea sonorae. Ind. Crop. Prod. 2015, 77, 895–900. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; López-Gresa, M.P.; Borsai, O.; Daniso, E.; Donat-Torres, M.P.; Mayoral, O.; Vicente, O.; Boscaiu, M. Native-Invasive Plants vs. Halophytes in Mediterranean Salt Marshes: Stress Tolerance Mechanisms in Two Related Species. Front. Plant Sci. 2016, 7, 473. [Google Scholar] [CrossRef] [Green Version]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 2014, 6, plu049. [Google Scholar] [CrossRef] [PubMed]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and Domesticating Crops Adapted to Drought and Salinity: A New Paradigm for Increasing Food Production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [Green Version]
- Harispe-Grandperrin, M.; Harispe, J.V. Proximate principles of Inula crithmoides L. family Compositae. I. Biochemical study of glucide constituents. Bull. Soc. Chim. Biol. 1943, 25, 416–418. [Google Scholar]
- Kacem, I.; Majdoub, H.; Roudesli, S. Fraction of soluble polysaccharides from Inula crithmoides by sequential extraction. J. Appl. Sci. 2008, 8, 2442–2448. [Google Scholar] [CrossRef]
- Attard, E.; Pacioni, P. The Phytochemical and In Vitro Pharmacological Testing of Maltese Medicinal Plants. In Bioactive Compounds in Phytomedicine; InTech: London, UK, 2011; pp. 93–112. [Google Scholar]
- Selim, M.A.; El-Hefnawy, H.M.; Ibrahim, T.A.; Sleem, A.A. Flavonoids and antihepatotoxic activity of Inula crithmoides L. growing in Egypt. Egypt. J. Biomed. Sci. 2003, 12, 83–95. [Google Scholar]
- El-Lakany, A.M.; Aboul Ela, M.A.; Hammoda, H.M.; Ghazy, N.M.; Mahmoud, Z.F. New methoxylated flavonols from Inula crithmoides. Pharmazie 1996, 51, 435–436. [Google Scholar]
- Metwally, M.A.; Dawidar, A.M. A thymol derivative from Inula crithmoides. Phytochemistry 1985, 24, 1377–1378. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz-Cervera, J.F.; Manglano, E. Chlorinated thymol derivatives from Inula crithmoides. Phytochemistry 1993, 33, 875–878. [Google Scholar] [CrossRef]
- Jdey, A.; Falleh, H.; Ben Jannet, S.; Mkadmini Hammi, K.; Dauvergne, X.; Ksouri, R.; Magne, C. Phytochemical investigation and antioxidant, antibacterial and anti-tyrosinase performances of six medicinal halophytes. S. Afr. J. Bot. 2017, 112, 508–514. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Grigore, A.; Pinto, D.C.G.A.; Silva, A.M.S. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.-W.; Qin, J.-J.; Cheng, X.-R.; Shen, Y.-H.; Shan, L.; Jin, H.-Z.; Zhang, W.-D. Inula sesquiterpenoids: Structural diversity, cytotoxicity and anti-tumor activity. Expert Opin. Investig. Drugs 2014, 23, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic Acids, Cytotoxic, Antioxidant, Acetylcholinesterase and Tyrosinase Enzyme Inhibitory Activities of Six Inula Species from Bulgaria. Chem. Biodivers. 2020. Ahead of Print. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Abdel-Azim, S.H.; El-Nekeety, A.A. Inula crithmoides extract protects against ochratoxin A-induced oxidative stress, clastogenic and mutagenic alterations in male rats. Toxicon 2008, 52, 566–573. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Neue norkauren- und thymol-derivate aus Athrixia-arten. Phytochemistry 1977, 16, 1773–1776. [Google Scholar] [CrossRef]
- Pacciaroni, A.D.V.; Sosa, V.E.; Espinar, L.A.; Oberti, J.C. Acyl glucopyranosides and other constituents from Schkuhria multiflora. An. Asoc. Quim. Argent. 1998, 86, 256–260. [Google Scholar]
- Chen, J.-J.; Tsai, Y.-C.; Hwang, T.-L.; Wang, T.-C. Thymol, Benzofuranoid, and Phenylpropanoid Derivatives: Anti-inflammatory Constituents from Eupatorium cannabinum. J. Nat. Prod. 2011, 74, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Passreiter, C.M.; Matthiesen, U.; Willuhn, G. 10-Acetoxy-9-chloro-8,9-dehydrothymol and further thymol derivatives from Arnica sachalinensis. Phytochemistry 1998, 49, 777–778. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Wei, P.; Cheng, X.; Ren, J.; Yan, S.; Zhang, W.; Jin, H. Chemical constituents from Inula wissmanniana and their anti-inflammatory activities. Arch. Pharm. Res. 2013, 36, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-R.; Ye, J.; Ren, J.; Zeng, Q.; Zhang, F.; Qin, J.-J.; Shen, Y.-H.; Zhang, W.-D.; Jin, H.-Z. Terpenoids from Inula sericophylla Franch. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2012, 42, 75–78. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Liu, Q.; Gao, K. Antimicrobial activities of some thymol derivatives from the roots of Inula hupehensis. Food Chem. 2010, 120, 512–516. [Google Scholar] [CrossRef]
- Adorisio, S.; Fierabracci, A.; Muscari, I.; Liberati, A.M.; Cannarile, L.; Thuy, T.T.; Sung, T.V.; Sohrab, H.; Hasan, C.M.; Ayroldi, E.; et al. Fusarubin and Anhydrofusarubin Isolated from A Cladosporium Species Inhibit Cell Growth in Human Cancer Cell Lines. Toxins 2019, 11, 503. [Google Scholar] [CrossRef] [Green Version]
- Yami, A.; Hamzeloo-Moghadam, M.; Darbandi, A.; Karami, A.; Mashati, P.; Takhviji, V.; Gharehbaghian, A. Ergolide, a potent sesquiterpene lactone induces cell cycle arrest along with ROS-dependent apoptosis and potentiates vincristine cytotoxicity in ALL cell lines. J. Ethnopharmacol. 2020, 253, 112504. [Google Scholar] [CrossRef]
- Karami, A.; Hamzeloo-Moghadam, M.; Yami, A.; Barzegar, M.; Mashati, P.; Gharehbaghian, A. Antiproliferative Effect of Gaillardin from Inula oculus-christi in Human Leukemic Cells. Nutr. Cancer 2019, 1–14. [Google Scholar] [CrossRef]
- Xu, X.; Huang, L.; Zhang, Z.; Tong, J.; Mi, J.; Wu, Y.; Zhang, C.; Yan, H. Targeting non-oncogene ROS pathway by alantolactone in B cell acute lymphoblastic leukemia cells. Life Sci. 2019, 227, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, L.; Jiang, J.; Zhao, C.; Yang, C. Costunolide enhances sensitivity of K562/ADR chronic myeloid leukemia cells to doxorubicin through PI3K/Akt pathway. Phytother. Res. 2019, 33, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Meng, X.; Li, Y.; Yang, C.; Liu, Y. Growth inhibition effects of isoalantolactone on K562/A02 cells: Caspase-dependent apoptotic pathways, S phase arrest, and downregulation of Bcr/Abl. Phytother. Res. 2014, 28, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Sehar, I.; Bhushan, S.; Gupta, B.D.; Saxena, A.K. Activation of caspases and poly (ADP-ribose) polymerase cleavage to induce apoptosis in leukemia HL-60 cells by Inula racemosa. Toxicol. Vitr. 2010, 24, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.Z.; Tan, N.H.; Ji, C.J.; Fan, J.T.; Huang, H.Q.; Han, H.J.; Zhou, G.B. Apoptosis inducement of bigelovin from Inula helianthus-aquatica on human Leukemia U937 cells. Phytother. Res. 2009, 23, 885–891. [Google Scholar] [CrossRef]
- Pan, M.H.; Chiou, Y.S.; Cheng, A.C.; Bai, N.; Lo, C.Y.; Tan, D.; Ho, C.T. Involvement of MAPK, Bcl-2 family, cytochrome c, and caspases in induction of apoptosis by 1,6-O,O-diacetylbritannilactone in human leukemia cells. Mol. Nutr. Food Res. 2007, 51, 229–238. [Google Scholar] [CrossRef]
- Yue, G.G.; Chan, B.C.; Kwok, H.F.; Wong, Y.L.; Leung, H.W.; Ji, C.J.; Fung, K.P.; Leung, P.C.; Tan, N.H.; Lau, C.B. Anti-angiogenesis and immunomodulatory activities of an anti-tumor sesquiterpene bigelovin isolated from Inula helianthus-aquatica. Eur. J. Med. Chem. 2013, 59, 243–252. [Google Scholar] [CrossRef]
- Ding, Y.; Pan, W.; Xu, J.; Wang, T.; Chen, T.; Liu, Z.; Xie, C.; Zhang, Q. Sesquiterpenoids from the roots of Inula helenium inhibit acute myelogenous leukemia progenitor cells. Bioorg. Chem. 2019, 86, 363–367. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Uyanikgil, Y.; Ozturk, F. Apoptotic effects of thymol, a novel monoterpene phenol, on different types of cancer. Bratisl. Lek. Listy 2020, 121, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Liu, Y.; Shi, R.; Zhang, D.; Li, C.; Shi, J. New thymol and isothymol derivatives from Eupatorium fortunei and their cytotoxic effects. Bioorg. Chem. 2020, 103644. [Google Scholar] [CrossRef]
- De La Chapa, J.J.; Singha, P.K.; Lee, D.R.; Gonzales, C.B. Thymol inhibits oral squamous cell carcinoma growth via mitochondria-mediated apoptosis. J. Oral Pathol. Med. 2018, 47, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Gunes-Bayir, A.; Kocyigit, A.; Guler, E.M. In vitro effects of two major phenolic compounds from the family Lamiaceae plants on the human gastric carcinoma cells. Toxicol. Ind. Health 2018, 34, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.; Huong, N.T.T.; Nhung, L.T.H.; Ninh, P.T.; Delfino, D.V.; Van Sung, T. Isolation, characterization and biological evaluation of a phenoxazine, a natural dyestuff isolated from leaves of Peristrophe bivalvis. Nat. Prod. Res. 2013, 27, 771–774. [Google Scholar] [CrossRef]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adorisio, S.; Giamperi, L.; Bucchini, A.E.A.; Delfino, D.V.; Marcotullio, M.C. Bioassay-Guided Isolation of Antiproliferative Compounds from Limbarda crithmoides (L.) Dumort. Molecules 2020, 25, 1893. https://doi.org/10.3390/molecules25081893
Adorisio S, Giamperi L, Bucchini AEA, Delfino DV, Marcotullio MC. Bioassay-Guided Isolation of Antiproliferative Compounds from Limbarda crithmoides (L.) Dumort. Molecules. 2020; 25(8):1893. https://doi.org/10.3390/molecules25081893
Chicago/Turabian StyleAdorisio, Sabrina, Laura Giamperi, Anahi Elena Ada Bucchini, Domenico Vittorio Delfino, and Maria Carla Marcotullio. 2020. "Bioassay-Guided Isolation of Antiproliferative Compounds from Limbarda crithmoides (L.) Dumort" Molecules 25, no. 8: 1893. https://doi.org/10.3390/molecules25081893
APA StyleAdorisio, S., Giamperi, L., Bucchini, A. E. A., Delfino, D. V., & Marcotullio, M. C. (2020). Bioassay-Guided Isolation of Antiproliferative Compounds from Limbarda crithmoides (L.) Dumort. Molecules, 25(8), 1893. https://doi.org/10.3390/molecules25081893