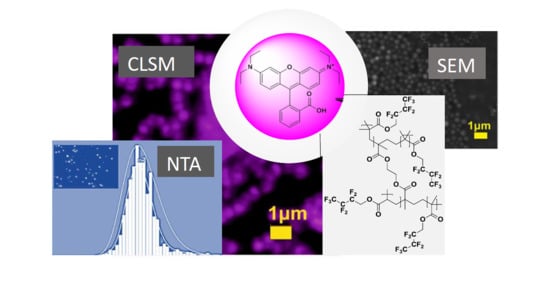
Fluorescent Submicron-Sized Poly(heptafluoro-n-butyl methacrylate) Particles with Long-Term Stability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Particle Size and Morphology Characterization
2.2. Spectroscopic and Contact Angle Analysis
X-ray Photoelectron Spectroscopy (XPS) and Contact Angle (CA) Analysis
2.3. Fluorescent Behavior Investigations
Fluorescent Behavior Investigations
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Methods
3.3.1. Hydrodynamic Diameter and Zeta Potential Determination
3.3.2. XPS
3.3.3. Contact Angle
3.3.4. Microscopic Investigations
3.3.5. Fluorescence and UV-VIS Spectroscopy
3.3.6. Fluorescence Lifetime
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khatami, M.; Alijani, H.Q.; Nejad, M.S.; Varma, R.S. Core@shell nanoparticles: Greener synthesis using natural plant products. Appl. Sci. 2018, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Chen, L. Effect of net surface charge on physical properties of the cellulose nanoparticles and their efficacy for oral protein delivery. Carbohydr. Polym. 2015, 121, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Śliwa, T.; Jarzębski, M. Dynamic Light Scattering Investigation of Pnipam-Co-Maa Microgel Solution. Curr. Top. Biophys. 2015, 37, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Zentel, R. Polymer coated semiconducting nanoparticles for hybrid materials. Inorganics 2020, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Jarzębski, M.; Fathordoobady, F.; Guo, Y.; Xu, M.; Singh, A.; Kitts, D.D.; Kowalczewski, P.Ł.; Jeżowski, P.; Pratap Singh, A. Pea Protein for Hempseed Oil Nanoemulsion Stabilization. Molecules 2019, 24, 4288. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, T.; Hanaichi, T.; Hasegawa, M.; Maruno, S. Dextran-magnetite complex: Conformation of dextran chains and stability of solution. J. Mater. Sci. Mater. Med. 2001, 12, 121–127. [Google Scholar] [CrossRef]
- García, R.S.; Stafford, S.; Gun’ko, Y.K. Recent progress in synthesis and functionalization of multimodal fluorescent-magnetic nanoparticles for biological applications. Appl. Sci. 2018, 8, 12–16. [Google Scholar]
- Feldman, D. Polymers and polymer nanocomposites for cancer therapy. Appl. Sci. 2019, 9, 3899. [Google Scholar] [CrossRef] [Green Version]
- Mastrodonato, C.; Pagano, P.; Daniel, J.; Vaultier, M.; Blanchard-Desce, M. Molecular-based fluorescent nanoparticles built from dedicated dipolar thienothiophene dyes as ultra-bright green to NIR nanoemitters. Molecules 2016, 21, 1277. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.C.C.; Mansur, A.A.P.; Mansur, H.S. One-step biofunctionalization of quantum dots with chitosan and n-palmitoyl chitosan for potential biomedical applications. Molecules 2013, 18, 6550–6572. [Google Scholar] [CrossRef] [Green Version]
- Gaihre, B.; Aryal, S.; Barakat, N.A.M.; Kim, H.Y. Gelatin stabilized iron oxide nanoparticles as a three dimensional template for the hydroxyapatite crystal nucleation and growth. Mater. Sci. Eng. C 2008, 28, 1297–1303. [Google Scholar] [CrossRef]
- Gaihre, B.; Khil, M.S.; Lee, D.R.; Kim, H.Y. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. Int. J. Pharm. 2009, 365, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Gaihre, B.; Aryal, S.; Khil, M.S.; Kim, H.Y. Encapsulation of Fe3O4 in gelatin nanoparticles: Effect of different parameters on size and stability of the colloidal dispersion. J. Microencapsul. 2008, 25, 21–30. [Google Scholar] [CrossRef]
- Haroon Anwar, S. A Brief Review on Nanoparticles: Types of Platforms, Biological Synthesis and Applications. Res. Rev. J. Mater. Sci. 2018, 6, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Jarzębski, M.; Śliwa, T.; Peplińska, B.; Jakubowicz, J.; Kuzioła, R.; Kościński, J.; Białopiotrowicz, T.; Gapiński, J. Submicron sized fluorescent silica particles characterization. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms 2017, 411, 78–84. [Google Scholar] [CrossRef]
- Jarzebski, M.; Zhang, Y.; Sliwa, T.; Mazuryk, J.; Deptula, T.; Kucinska, M.; Murias, M.; Buitenhuis, J.; Gapi?ski, J.; Patkowski, A. Core-shell fluorinated methacrylate nanoparticles with Rhodamine-B for confocal microscopy and fluorescence correlation spectroscopy applications. J. Fluor. Chem. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [Green Version]
- Cheruku, S.; D’Olieslaeger, L.; Smisdom, N.; Smits, J.; Vanderzande, D.; Maes, W.; Ameloot, M.; Ethirajan, A. Fluorescent PCDTBT nanoparticles with tunable size for versatile bioimaging. Materials 2019, 12, 2497. [Google Scholar] [CrossRef] [Green Version]
- Šamec, N.; Zottel, A.; Paska, A.V.; Jovčevska, I. Nanomedicine and immunotherapy: A step further towards precision medicine for glioblastoma. Molecules 2020, 25, 490. [Google Scholar] [CrossRef] [Green Version]
- Genevois, C.; Hocquelet, A.; Mazzocco, C.; Rustique, E.; Couillaud, F.; Grenier, N. In vivo imaging of prostate cancer tumors and metastasis using non-specific fluorescent nanoparticles in mice. Int. J. Mol. Sci. 2017, 18, 2584. [Google Scholar] [CrossRef] [Green Version]
- Jarzębski, M.; Peplińska, B.; Florczak, P.; Gapiński, J.; Flak, D.; Mała, P.; Ramanavicius, A.; Baryła-Pankiewicz, E.; Kobus- Cisowska, J.; Szwajca, A. Fluorescein ether-ester dyes for labeling of fluorinated methacrylate nanoparticles. J. Photochem. Photobiol. A Chem. 2019, 382, 111956. [Google Scholar] [CrossRef]
- Gapinski, J.; Jarzębski, M.; Buitenhuis, J.; Deptula, T.; Mazuryk, J.; Patkowski, A. Structure and Dimensions of Core-Shell Nanoparticles Comparable to the Confocal Volume Studied by Means of Fluorescence Correlation Spectroscopy. Langmuir 2016, 32, 2482–2491. [Google Scholar] [CrossRef] [PubMed]
- Deptuła, T.; Buitenhuis, J.; Jarzebski, M.; Patkowski, A.; Gapinski, J. Size of Submicrometer Particles Measured by FCS: Correction of the Confocal Volume. Langmuir 2015, 31, 6681–6687. [Google Scholar] [CrossRef] [PubMed]
- Tavernaro, I.; Cavelius, C.; Peuschel, H.; Kraegeloh, A. Bright fluorescent silica-nanoparticle probes for high-resolution STED and confocal microscopy. Beilstein J. Nanotechnol. 2017, 8, 1283–1296. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.Y.; Debele, T.A.; Kao, Y.C.; Tsai, H.C. Synthesis and characterization of dual-sensitive fluorescent nanogels for enhancing drug delivery and tracking intracellular drug delivery. Int. J. Mol. Sci. 2017, 18, 1090. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, G.; Wang, J.; Sun, Q.; Jian, X.; Teng, J.; Zhang, H. Fluorinated poly(phthalazinone ether)s with tunable refractive index: Synthesis, characterization and optical properties. Polym. J. 2008, 40, 92–93. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Kasuya, M.C.; Hatanaka, K.; Matsuoka, K. Synthesis of fluorinated polymers and evaluation of wettability. Molecules 2016, 21, 358. [Google Scholar] [CrossRef] [Green Version]
- Sabatini, V.; Pargoletti, E.; Comite, V.; Ortenzi, M.A.; Fermo, P.; Gulotta, D.; Cappelletti, G. Towards novel fluorinated methacrylic coatings for cultural heritage: A combined polymers and surfaces chemistry study. Polymers 2019, 11, 1190. [Google Scholar] [CrossRef] [Green Version]
- Masotti, E.; Poma, N.; Guazzelli, E.; Fiaschi, I.; Glisenti, A.; Vivaldi, F.; Bonini, A.; Francesco, F.D.; Tavanti, A.; Galli, G.; et al. Fluorinated vs. zwitterionic-polymer grafted surfaces for adhesion prevention of the fungal pathogen Candida albicans. Polymers 2020, 12, 398. [Google Scholar] [CrossRef] [Green Version]
- Iacono, S.T.; Jennings, A.R. Recent studies on fluorinated silica nanometer-sized particles. Nanomaterials 2019, 9, 684. [Google Scholar] [CrossRef] [Green Version]
- Hequet; Henoumont; Djouana Kenfack; Lemaur; Lazzaroni; Boutry; Elst; Muller; Laurent Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI. Magnetochemistry 2020, 6, 8. [CrossRef]
- Antonova, I.; Nebogatikova, N.; Zerrouki, N.; Kurkina, I.; Ivanov, A. Flexibility of fluorinated graphene-based materials. Materials 2020, 13, 1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaszuba, M.; McKnight, D.; Connah, M.T.; McNeil-Watson, F.K.; Nobbmann, U. Measuring sub nanometre sizes using dynamic light scattering. J. Nanoparticle Res. 2008, 10, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Bell, N.C.; Minelli, C.; Tompkins, J.; Stevens, M.M.; Shard, A.G. Emerging Techniques for Submicrometer Particle Sizing Applied to Stöber Silica. Langmuir 2012, 28, 10860–10872. [Google Scholar] [CrossRef] [PubMed]
- Jarzȩbski, M.; Kościński, M.; Białopiotrowicz, T. Determining the size of nanoparticles in the example of magnetic iron oxide core-shell systems. J. Phys. Conf. Ser. 2017, 885. [Google Scholar] [CrossRef]
- Khmelinskaia, A.; Mücksch, J.; Conci, F.; Chwastek, G.; Schwille, P. FCS Analysis of Protein Mobility on Lipid Monolayers. Biophys. J. 2018, 114, 2444–2454. [Google Scholar] [CrossRef] [Green Version]
- Banachowicz, E.; Patkowski, A.; Meier, G.; Klamecka, K.; Gapiński, J. Successful FCS experiment in nonstandard conditions. Langmuir 2014, 30, 8945–8955. [Google Scholar] [CrossRef]
- Nägele, G.; Dhont, J.K.G.; Meier, G. Diffusion in Colloidal and Polymeric Systems. In Diffusion in Condensed Matter; Heitjans, P., Karger, J., Eds.; Springer: Berlin/Heidelberg, Germany; pp. 619–715. ISBN 978-3-540-20043-7.
- Kubin, R.F.; Fletcher, A.N. Fluorescence quantum yields of some rhodamine dyes. J. Lumin. 1982, 27, 455–462. [Google Scholar] [CrossRef]
- Guldbrand, S.; Kirejev, V.; Simonsson, C.; Goksör, M.; Smedh, M.; Ericson, M.B. Two-photon fluorescence correlation spectroscopy as a tool for measuring molecular diffusion within human skin. Eur. J. Pharm. Biopharm. 2013, 84, 430–436. [Google Scholar] [CrossRef]
- Rurack, K. Fluorescence Quantum Yields: Methods of Determination and Standards; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-75207-3. [Google Scholar]
- Liu, W.; Wilson, D.I.; Chen, X.D.; Mercadé-Prieto, R. Quantification of the Local Protein Content in Hydrogels Undergoing Swelling and Dissolution at Alkaline pH Using Fluorescence Microscopy. Food Bioprocess Technol. 2018, 11, 572–584. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, J.T.; Kim, C.B.; Shin, Y.R.; Park, P.G.; Kim, H.; Lee, J.M.; Park, J.H. Microneedle Array Patch (MAP) Consisting of Crosslinked Hyaluronic Acid Nanoparticles for Processability and Sustained Release. Pharm. Res. 2020, 37, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Gómez, S.; Campanero, M.A.; Gamazo, C.; Irache, J.M. Bioadhesive properties of pegylated nanoparticles. Expert Opin. Drug Deliv. 2005, 2, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cui, C.; Wang, L.; Lei, J.; Zhang, J. Dual-Shell Fluorescent Nanoparticles for Self-Monitoring of pH-Responsive Molecule-Releasing in a Visualized Way. ACS Appl. Mater. Interfaces 2016, 8, 19084–19091. [Google Scholar] [CrossRef]
- Sacanna, S.; Koenderink, G.H.; Philipse, A.P. Microemulsion Synthesis of Fluorinated Latex Spheres. Langmuir 2004, 20, 8398–8400. [Google Scholar] [CrossRef] [Green Version]
- Koenderink, G.H.; Sacanna, S.; Pathmamanoharan, C.; Ras, M.; Philipse, A.P. Preparation and Properties of Optically Transparent Aqueous Dispersions of Monodisperse Fluorinated Colloids. Langmuir 2001, 17, 6086–6093. [Google Scholar] [CrossRef]
- Carpenter, D.K. Dynamic light scattering: with applications to chemistry, biology, and physics (Berne, B.J.; Pecora, R.). J. Chem. Educ. 1977, 54, A430. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds (HFBMA NPs) are available from the authors. |












| Sample | Description |
|---|---|
| H1 | Transparent core |
| H2 ibH8 | Transparent core with a transparent shell ibFluorescent core with a transparent shell |
| H10, H14, H16, H22 | Fluorescent core |
| H26, H27, H36 | Fluorescent core with a transparent shell |
| RBITC | Rhodamine-B-isothiocyanate |
| Sample | Time | ||||||
|---|---|---|---|---|---|---|---|
| As-Prepared Samples * | Six Years of Storage ** | Seven Years of Storage ** | |||||
| Average Diameter d (nm) | z-ave (nm) | pdi | Mean Peak (nm) | z-ave (nm) | pdi | Mean Peak (nm) | |
| H1 | 184 | 219 | 0.022 | 228 | 223 | 0.014 | 232 |
| H2 | 532 | 305 | 0.037 | 316 | 302 | 0.039 | 315 |
| H10 | 188 | 248 | 0.033 | 260 | 250 | 0.022 | 261 |
| H14 | 326 | 348 | 0.041 | 362 | 366 | 0.036 | 385 |
| H26 | 550 | 592 | 0.054 | 618 | 570 | 0.046 | 586 |
| Sample | Hydrodynamic Diameter (nm) | ||||
|---|---|---|---|---|---|
| DLS | NTA (mean) | NTA (mode) | FCS | ξ (mV) | |
| H1 | 184 | --- | --- | --- | −61.7 |
| H2 | 532 | --- | --- | --- | −71.6 |
| H10 | 188 | 218 | 209 | 236 | −71.2 |
| H14 | 326 | 338 | 328 | 306 | −63.8 |
| H26 | 550 | 579 | 556 | 508 | −67.8 |
| H27 | 540 | 539 | 514 | 490 | −72.3 |
| Sample | τdiff [ms] | Rapp = Rr × τ/τr | Rcorr | Correction Factor |
|---|---|---|---|---|
| RBITC | 0.0911 | 0.568 | 0.568 | 1.00 |
| H10 | 20.14 | 126 | 116 | 1.08 |
| H14 | 28.16 | 175 | 153 | 1.15 |
| H26 | 57.64 | 359 | 254 | 1.41 |
| H27 | 54.44 | 339 | 245 | 1.38 |
| Sample | C 1s | O 1s | |||||
|---|---|---|---|---|---|---|---|
| C–H | C–O | C=O | C–CF2 | C–CF3 | C–O | C=O | |
| H16 | 284.4 eV | +1.9 | +4.3 | +8.9 | +11.5 | 531.0 eV | +1.2 |
| H27 | 284.4 eV | +1.0 | +4.4 | +8.7 | +11.7 | 531.3 eV | +1.0 |
| Sample | λ (nm) | Fluorescence Band Shift (nm) | |
|---|---|---|---|
| Excitation | Emission | ||
| RBITC | 559 | 573 | 14 |
| H10 | 555 | 573 | 18 |
| H14 | 555 | 569 | 14 |
| H26 | 555 | 565 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarzębski, M.; Siejak, P.; Przeor, M.; Gapiński, J.; Woźniak, A.; Baranowska, H.M.; Pawlicz, J.; Baryła-Pankiewicz, E.; Szwajca, A. Fluorescent Submicron-Sized Poly(heptafluoro-n-butyl methacrylate) Particles with Long-Term Stability. Molecules 2020, 25, 2013. https://doi.org/10.3390/molecules25092013
Jarzębski M, Siejak P, Przeor M, Gapiński J, Woźniak A, Baranowska HM, Pawlicz J, Baryła-Pankiewicz E, Szwajca A. Fluorescent Submicron-Sized Poly(heptafluoro-n-butyl methacrylate) Particles with Long-Term Stability. Molecules. 2020; 25(9):2013. https://doi.org/10.3390/molecules25092013
Chicago/Turabian StyleJarzębski, Maciej, Przemysław Siejak, Monika Przeor, Jacek Gapiński, Anna Woźniak, Hanna Maria Baranowska, Jarosław Pawlicz, Elżbieta Baryła-Pankiewicz, and Anna Szwajca. 2020. "Fluorescent Submicron-Sized Poly(heptafluoro-n-butyl methacrylate) Particles with Long-Term Stability" Molecules 25, no. 9: 2013. https://doi.org/10.3390/molecules25092013
APA StyleJarzębski, M., Siejak, P., Przeor, M., Gapiński, J., Woźniak, A., Baranowska, H. M., Pawlicz, J., Baryła-Pankiewicz, E., & Szwajca, A. (2020). Fluorescent Submicron-Sized Poly(heptafluoro-n-butyl methacrylate) Particles with Long-Term Stability. Molecules, 25(9), 2013. https://doi.org/10.3390/molecules25092013