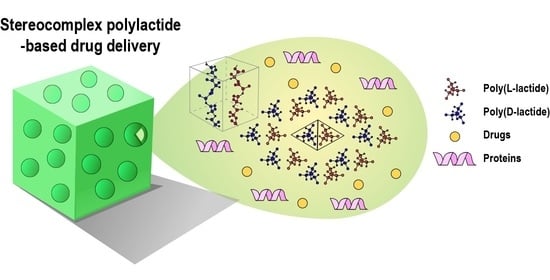
Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review
Abstract
:1. Introduction
2. Drug Delivery
2.1. Stereocomplexed Micelle System
2.2. Self-Assembled Nanoparticle
2.3. Emulsion Blending
2.4. Inkjet Printing
2.5. Stereocomplex Hydrogel
3. Biomedical Applications
3.1. Anti-Cancer Therapy
3.2. Tissue Engineering
3.3. Anti-Microbial Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Im, S.H.; Park, S.J.; Chung, J.J.; Jung, Y.; Kim, S.H. Creation of polylactide vascular scaffolds with high compressive strength using a novel melt-tube drawing method. Polymer 2019, 166, 130–137. [Google Scholar] [CrossRef]
- Im, S.H.; Jung, Y.; Kim, S.H. Current status and future direction of biodegradable metallic and polymeric vascular scaffolds for next-generation stents. Acta Biomater. 2017, 60, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly (lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Tsuji, H.; Hyon, S.H.; Ikada, Y. Stereocomplex formation between enantiomeric poly (lactic acid) s. 4. Differential scanning calorimetric studies on precipitates from mixed solutions of poly (D-lactic acid) and poly (L-lactic acid). Macromolecules 1991, 24, 5657–5662. [Google Scholar] [CrossRef]
- Im, S.H.; Lee, C.W.; Bibi, G.; Jung, Y.; Kim, S.H. Supercritical fluid technology parameters affecting size and behavior of stereocomplex polylactide particles and their composites. Polym Eng. Sci. 2018, 58, 1193–1200. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly (lactic acid) s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar]
- Deng, S.; Bai, H.; Liu, Z.; Zhang, Q.; Fu, Q. Toward supertough and heat-resistant stereocomplex-type polylactide/elastomer blends with impressive melt stability via in situ formation of graft copolymer during one-pot reactive melt blending. Macromolecules 2019, 52, 1718–1730. [Google Scholar] [CrossRef]
- Bibi, G.; Jung, Y.; Lim, J.-C.; Kim, S.H. Novel strategy of lactide polymerization leading to stereocomplex polylactide nanoparticles using supercritical fluid technology. ACS Sustain. Chem. Eng. 2016, 4, 4521–4528. [Google Scholar] [CrossRef]
- Im, S.H.; Jung, Y.; Kim, S.H. In situ homologous polymerization of l-lactide Having a stereocomplex crystal. Macromolecules 2018, 51, 6303–6311. [Google Scholar]
- Luo, F.; Fortenberry, A.; Ren, J.; Qiang, Z.J. Recent progress in enhancing poly (lactic acid) stereocomplex formation for material property improvement. Front. Chem. 2020, 8, 688. [Google Scholar]
- Sánchez, A.; Mejía, S.P.; Orozco, J. Recent Advances in Polymeric Nanoparticle-Encapsulated Drugs against Intracellular Infections. Molecules 2020, 25, 3760. [Google Scholar] [CrossRef]
- Tsuji, H. Poly (lactic acid) stereocomplexes: A decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Scheuer, K.; Bandelli, D.; Helbing, C.; Weber, C.; Alex, J.; Max, J.B.; Hocken, A.; Stranik, O.; Seiler, L.; Gladigau, F. Self-assembly of copolyesters into stereocomplex crystallites tunes the properties of polyester nanoparticles. Macromolecules 2020, 53, 8340–8351. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, D.; Jin, G.; Tan, B.H.; He, C. Facile layer-by-layer self-assembly toward enantiomeric poly (lactide) stereocomplex coated magnetite nanocarrier for highly tunable drug deliveries. ACS Appl. Mater. Interfaces 2016, 8, 1842–1853. [Google Scholar] [CrossRef]
- Boi, S.; Dellacasa, E.; Bianchini, P.; Petrini, P.; Pastorino, L.; Monticelli, O.J.C. Encapsulated functionalized stereocomplex PLA particles: An effective system to support mucolytic enzymes. Colloids Surf. B Colloid Surface B 2019, 179, 190–198. [Google Scholar] [CrossRef]
- Niu, K.; Yao, Y.; Xiu, M.; Guo, C.; Ge, Y.; Wang, J. Controlled drug delivery by polylactide stereocomplex micelle for cervical cancer chemotherapy. Front. Pharmacol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Li, W.; Fan, X.; Wang, X.; Shang, X.; Wang, Q.; Lin, J.; Hu, Z.; Li, Z. Stereocomplexed micelle formation through enantiomeric PLA-based Y-shaped copolymer for targeted drug delivery. Mater. Sci. Eng. C 2018, 91, 688–695. [Google Scholar] [CrossRef]
- Burt, H.M.; Zhang, X.; Toleikis, P.; Embree, L.; Hunter, W.L. Development of copolymers of poly (D, L-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf. B Colloid Surface B 1999, 16, 161–171. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.-C.; Leroux, J.-C. Polymeric micelles–a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111. [Google Scholar] [CrossRef]
- Kang, N.; Perron, M.-È.; Prud’Homme, R.E.; Zhang, Y.; Gaucher, G.; Leroux, J.-C. Stereocomplex block copolymer micelles: Core− shell nanostructures with enhanced stability. Nano Lett. 2005, 5, 315–319. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Cao, M.; Zhang, X.; Lin, J.; Tian, Q.; Yang, S. PH and glutathione synergistically triggered release and self-assembly of Au nanospheres for tumor theranostics. ACS Appl. Mater. Interfaces 2020, 12, 8050–8061. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zhu, X.; Zhang, J.; Zhao, Y. Temperature and solvent isotope dependent hierarchical self-assembly of a heterografted block copolymer. Chem. Commun. 2019, 55, 5709–5712. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Dong, S.; Kuchel, R.P.; Perrier, S.; Zetterlund, P.B. Polymerization induced self-assembly: Tuning of morphology using ionic strength and pH. Polym. Chem. 2017, 8, 3082–3089. [Google Scholar]
- Gerbelli, B.B.; Vassiliades, S.V.; Rojas, J.E.; Pelin, J.N.; Mancini, R.S.; Pereira, W.S.; Aguilar, A.M.; Venanzi, M.; Cavalieri, F.; Giuntini, F. Hierarchical self-assembly of peptides and its applications in bionanotechnology. Macromol. Chem. Phys. 2019, 220, 1900085. [Google Scholar] [CrossRef]
- Bishara, A.; Kricheldorf, H.R.; Domb, A.J. Stereocomplexes of Triblock Poly (lactide-PEG2000-lactide) as Carrier of Drugs. Macromol. Symp. 2005, 225, 17–30. [Google Scholar] [CrossRef]
- Liu, R.; He, B.; Li, D.; Lai, Y.; Tang, J.Z.; Gu, Z. Stabilization of pH-Sensitive mPEG–PH–PLA nanoparticles by stereocomplexation between enantiomeric polylactides. Macromol. Rapid Commun. 2012, 33, 1061–1066. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005, 5, 325–329. [Google Scholar] [CrossRef]
- Lim, D.W.; Park, T.G. Stereocomplex formation between enantiomeric PLA–PEG–PLA triblock copolymers: Characterization and use as protein-delivery microparticulate carriers. J. App. Polym. Sci. 2000, 75, 1615–1623. [Google Scholar] [CrossRef]
- Kurapati, R.; Groth, T.W.; Raichur, A.M. Recent developments in layer-by-layer technique for drug delivery applications. ACS Appl. Bio Mater. 2019, 2, 5512–5527. [Google Scholar] [CrossRef]
- Hellwig, J.; Strebe, J.; Klitzing, R.V. Effect of environmental parameters on the nano mechanical properties of hyaluronic acid/poly (l-lysine) multilayers. Polymers 2018, 20, 19082–19086. [Google Scholar] [CrossRef]
- Apte, G.; Repanas, A.; Willems, C.; Mujtaba, A.; Schmelzer, C.E.; Raichur, A.; Syrowatka, F.; Groth, T. Effect of different crosslinking strategies on physical properties and biocompatibility of freestanding multilayer films made of alginate and chitosan. Macromol. Biosci. 2019, 19, 1900181. [Google Scholar] [CrossRef]
- Luo, X.; Song, H.; Yang, J.; Han, B.; Feng, Y.; Leng, Y.; Chen, Z. Encapsulation of Escherichia coli strain Nissle 1917 in a chitosan―alginate matrix by combining layer-by-layer assembly with CaCl2 cross-linking for an effective treatment of inflammatory bowel diseases. Colloids Surf. B Colloid Surf. B 2020, 189, 110818. [Google Scholar] [CrossRef]
- Ariga, K.; Ahn, E.; Park, M.; Kim, B.S. Layer-by-layer assembly: Recent progress from layered assemblies to layered nanoarchitectonics. Chem. Asian J. 2019, 14, 2553–2566. [Google Scholar] [CrossRef]
- Kondo, K.; Kida, T.; Ogawa, Y.; Arikawa, Y.; Akashi, M.J. Nanotube formation through the continuous one-dimensional fusion of hollow nanocapsules composed of layer-by-layer poly (lactic acid) stereocomplex films. J. Am. Chem. Soc. 2010, 132, 8236–8237. [Google Scholar] [CrossRef]
- Ahvenniemi, E.; Karppinen, M. In Situ atomic/molecular layer-by-layer deposition of inorganic–organic coordination network thin films from gaseous precursors. Chem. Mater. 2016, 28, 6260–6265. [Google Scholar] [CrossRef] [Green Version]
- Dellacasa, E.; Zhao, L.; Yang, G.; Pastorino, L.; Sukhorukov, G.B. Fabrication and characterization of novel multilayered structures by stereocomplexion of poly (D-lactic acid)/poly (L-lactic acid) and self-assembly of polyelectrolytes. Beilstein J. Nanotechnol. 2016, 7, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Brzeziński, M. Hollow microcapsules with enhanced stability via stereocomplex assemblies. Macromol. Chem. Phys. 2017, 218, 1700018. [Google Scholar] [CrossRef]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Yow, H.N.; Routh, A.F. Formation of liquid core–polymer shell microcapsules. Soft Matter 2006, 2, 940–949. [Google Scholar] [CrossRef]
- Ma, Q.; Song, Y.; Kim, J.W.; Choi, H.S.; Shum, H.C. Affinity partitioning-induced self-assembly in aqueous two-phase systems: Templating for polyelectrolyte microcapsules. ACS Macro Lett. 2016, 5, 666–670. [Google Scholar] [CrossRef]
- Liu, H.; Bai, D.; Bai, H.; Zhang, Q.; Fu, Q. Constructing stereocomplex structures at the interface for remarkably accelerating matrix crystallization and enhancing the mechanical properties of poly (L-lactide)/multi-walled carbon nanotube nanocomposites. J. Mater. Chem. A 2015, 3, 13835–13847. [Google Scholar] [CrossRef]
- Im, S.H.; Park, S.J.; Jung, Y.; Chung, J.J.; Kim, S.H. Strategy for stereocomplexation of polylactide using O/W emulsion blending and applications as composite fillers, drug carriers, and self-nucleating agents. ACS Sustain. Chem. Eng. 2020, 8, 8752–8761. [Google Scholar] [CrossRef]
- Akagi, T.; Fujiwara, T.; Akashi, M. Rapid Fabrication of Polylactide Stereocomplex Using Layer-by-Layer Deposition by Inkjet Printing. Angew. Chem. 2012, 124, 5589–5592. [Google Scholar] [CrossRef]
- Akagi, T.; Fujiwara, T.; Akashi, M. Inkjet printing of layer-by-layer assembled poly (lactide) stereocomplex with encapsulated proteins. Langmuir 2014, 30, 1669–1676. [Google Scholar] [CrossRef]
- Tran, H.T.; Ajiro, H.; Hsiao, Y.-J.; Akashi, M. Thermally resistant polylactide layer-by-layer film prepared using an inkjet approach. Polym. J. 2017, 49, 327–334. [Google Scholar] [CrossRef]
- De Jong, S.; Van Eerdenbrugh, B.; van Nostrum, C.V.; Kettenes-Van Den Bosch, J.; Hennink, W. Physically crosslinked dextran hydrogels by stereocomplex formation of lactic acid oligomers: Degradation and protein release behavior. J. Control. Release 2001, 71, 261–275. [Google Scholar] [CrossRef]
- Hennink, W.; De Jong, S.; Bos, G.; Veldhuis, T.; Van Nostrum, C. Biodegradable dextran hydrogels crosslinked by stereocomplex formation for the controlled release of pharmaceutical proteins. Int. J. Pharm. 2004, 277, 99–104. [Google Scholar] [CrossRef]
- Cao, L.; Cao, B.; Lu, C.; Wang, G.; Yu, L.; Ding, J. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J. Mater. Chem. B 2015, 3, 1268–1280. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Young, S.; Klouda, L.; Wong, M.; Mikos, A.G. Injectable biomaterials for regenerating complex craniofacial tissues. Adv. Mater. 2009, 21, 3368–3393. [Google Scholar] [CrossRef] [Green Version]
- Purcell, B.P.; Lobb, D.; Charati, M.B.; Dorsey, S.M.; Wade, R.J.; Zellars, K.N.; Doviak, H.; Pettaway, S.; Logdon, C.B.; Shuman, J.A. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat. Mater. 2014, 13, 653–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Cao, Y.; Zhao, H.; Zhang, L.; Ni, T.; Liu, Y.; An, Z.; Liu, M.; Pei, R.J. An injectable BMSC-laden enzyme-catalyzed crosslinking collagen-hyaluronic acid hydrogel for cartilage repair and regeneration. J. Mater. Chem. B 2020, 8, 4237–4244. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.-x.; Mo, X.-m.; Pan, J.-F. In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Zhao, Z.; Si, D.; Zhou, H.; Yang, M.; Wang, X. An In situ forming hydrogel based on photo-induced hydrogen bonding. Macromol. Res. 2020, 28, 1127–1133. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef]
- Goos, J.A.; Cho, A.; Carter, L.M.; Dilling, T.R.; Davydova, M.; Mandleywala, K.; Puttick, S.; Gupta, A.; Price, W.S.; Quinn, J.F. Delivery of polymeric nanostars for molecular imaging and endoradiotherapy through the enhanced permeability and retention (EPR) effect. Theranostics 2020, 10, 567. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H.J. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, Y.; Yang, W.; Niu, P.; Li, X.; Chen, Y.; Li, Z.; Liu, Y.; An, Y.; Liu, Y. Investigating the EPR effect of nanomedicines in human renal tumors via ex vivo perfusion strategy. Nano Today 2020, 35, 100970. [Google Scholar] [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Guo, Z.; Sui, J.; Ma, M.; Hu, J.; Sun, Y.; Yang, L.; Fan, Y.; Zhang, X.J. pH-Responsive charge switchable PEGylated ε-poly-l-lysine polymeric nanoparticles-assisted combination therapy for improving breast cancer treatment. J. Control. Release 2020, 326, 350–364. [Google Scholar] [CrossRef]
- Goldberg, J.S. Stereocomplexes formed from select oligomers of polymer d-lactic acid (pDLA) and l-lactate may inhibit growth of cancer cells and help diagnose aggressive cancers—Applications of the Warburg effect. Perspect. Med. Chem. 2011, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brzeziński, M.; Kost, B.; Wedepohl, S.; Socka, M.; Biela, T.; Calderón, M. Stereocomplexed PLA microspheres: Control over morphology, drug encapsulation and anticancer activity. Colloids Surf. B Colloid Surf. B 2019, 184, 110544. [Google Scholar] [CrossRef]
- Kost, B.; Brzeziński, M.; Cieślak, M.; Królewska-Golińska, K.; Makowski, T.; Socka, M.; Biela, T.J. Stereocomplexed micelles based on polylactides with β-cyclodextrin core as anti-cancer drug carriers. Eur. Polym. J. 2019, 120, 109271. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.F. Challenges with the development of biomaterials for sustainable tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Polymers 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef] [PubMed]
- Sartore, L.; Inverardi, N.; Pandini, S.; Bignotti, F.; Chiellini, F. PLA/PCL-based foams as scaffolds for tissue engineering applications. Mater. Today Proc. 2019, 7, 410–417. [Google Scholar] [CrossRef]
- Mader, M.; Jérôme, V.R.; Freitag, R.; Agarwal, S.; Greiner, A. Ultraporous, compressible, wettable polylactide/polycaprolactone sponges for tissue engineering. Biomacromolecules 2018, 19, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Gayer, C.; Ritter, J.; Bullemer, M.; Grom, S.; Jauer, L.; Meiners, W.; Pfister, A.; Reinauer, F.; Vučak, M.; Wissenbach, K. Development of a solvent-free polylactide/calcium carbonate composite for selective laser sintering of bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 101, 660–673. [Google Scholar] [CrossRef]
- Gritsch, L.; Conoscenti, G.; La Carrubba, V.; Nooeaid, P.; Boccaccini, A.R. Polylactide-based materials science strategies to improve tissue-material interface without the use of growth factors or other biological molecules. Mater. Sci. Eng. C 2019, 94, 1083–1101. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, P.; Shi, X.; Zhang, G.; Wang, C. Multilevel structural stereocomplex polylactic acid/collagen membranes by pattern electrospinning for tissue engineering. Polymer 2018, 156, 250–260. [Google Scholar] [CrossRef]
- Im, S.H.; Kim, C.Y.; Lee, C.W.; Jung, Y.; Kim, S.H. Strategy for securing key patents in the field of biomaterials. Macromol. Res. 2020, 28, 87–98. [Google Scholar] [CrossRef]
- Gupta, A.; Prasad, A.; Mulchandani, N.; Shah, M.; Ravi Sankar, M.; Kumar, S.; Katiyar, V. Multifunctional nanohydroxyapatite-promoted toughened high-molecular-weight stereocomplex poly (lactic acid)-based bionanocomposite for both 3D-printed orthopedic implants and high-temperature engineering applications. ACS Omega 2017, 2, 4039–4052. [Google Scholar] [CrossRef]
- Mulchandani, N.; Gupta, A.; Masutani, K.; Kumar, S.; Sakurai, S.; Kimura, Y.; Katiyar, V. Effect of block length and stereocomplexation on the thermally processable poly (ε-caprolactone) and poly (lactic acid) block copolymers for biomedical applications. ACS Appl. Polym. Mater. 2019, 1, 3354–3365. [Google Scholar] [CrossRef]
- Singh, A.A.; Wei, J.; Herrera, N.; Geng, S.; Oksman, K. Synergistic effect of chitin nanocrystals and orientations induced by solid-state drawing on PLA-based nanocomposite tapes. Compos. Sci. Technol. 2018, 162, 140–145. [Google Scholar] [CrossRef]
- Lin, Y.; Tu, W.; Verpaalen, R.C.; Zhang, H.; Bastiaansen, C.W.; Peijs, T. Transparent, lightweight, and high strength polyethylene films by a scalable continuous extrusion and solid-state drawing process. Macromol. Mater. Eng. 2019, 304, 1900138. [Google Scholar] [CrossRef]
- Walker, J.; Melaj, M.; Giménez, R.; Pérez, E.; Bernal, C. Solid-state drawing of commercial poly (lactic acid)(PLA) based filaments. Front. Mater. 2019, 6, 280. [Google Scholar] [CrossRef] [Green Version]
- Im, S.H.; Jung, Y.; Jang, Y.; Kim, S.H. Poly (L-lactic acid) scaffold with oriented micro-valley surface and superior properties fabricated by solid-state drawing for blood-contact biomaterials. Biofabrication 2016, 8, 045010. [Google Scholar] [CrossRef]
- Im, S.H.; Kim, C.Y.; Jung, Y.; Jang, Y.; Kim, S.H. Biodegradable vascular stents with high tensile and compressive strength: A novel strategy for applying monofilaments via solid-state drawing and shaped-annealing processes. Biomater. Sci. 2017, 5, 422–431. [Google Scholar] [CrossRef]
- Li, J.; Ye, W.; Fan, Z.; Cao, L. A novel stereocomplex poly (lactic acid) with shish-kebab crystals and bionic surface structures as bioimplant materials for tissue engineering applications. ACS Appl. Mater. Interfaces 2021, 13, 5469–5477. [Google Scholar] [CrossRef]
- Wang, C.; Feng, N.; Chang, F.; Wang, J.; Yuan, B.; Cheng, Y.; Liu, H.; Yu, J.; Zou, J.; Ding, J. Injectable cholesterol-enhanced stereocomplex polylactide thermogel loading chondrocytes for optimized cartilage regeneration. Adv. Healthc. Mater. 2019, 8, 1900312. [Google Scholar] [CrossRef]
- Xue, Q.; Liu, X.-B.; Lao, Y.-H.; Wu, L.-P.; Wang, D.; Zuo, Z.-Q.; Chen, J.-Y.; Hou, J.; Bei, Y.-Y.; Wu, X.-F. Anti-infective biomaterials with surface-decorated tachyplesin I. Biomaterials 2018, 178, 351–362. [Google Scholar] [CrossRef]
- Reigada, I.; Pérez-Tanoira, R.; Patel, J.Z.; Savijoki, K.; Yli-Kauhaluoma, J.; Kinnari, T.J.; Fallarero, A. Strategies to prevent biofilm infections on biomaterials: Effect of novel naturally-derived biofilm inhibitors on a competitive colonization model of titanium by Staphylococcus aureus and SaOS-2 cells. Microorganisms 2020, 8, 345. [Google Scholar] [CrossRef] [Green Version]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Danese, P.N. Antibiofilm approaches: Prevention of catheter colonization. Chem. Biol. 2002, 9, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef]
- Oliveira, W.; Silva, P.; Silva, R.; Silva, G.; Machado, G.; Coelho, L.; Correia, M.J. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, J.-O.; Lee, S.; Park, J.-S.; Gwon, H.-J.; Jeong, S.I.; Hardy, J.G.; Lim, Y.-M.; Lee, J.Y. Effective gamma-ray sterilization and characterization of conductive polypyrrole biomaterials. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Harrington, R.E.; Guda, T.; Lambert, B.; Martin, J. Sterilization and disinfection of biomaterials for medical devices. Biomater. Sci. 2020, 1431–1446. [Google Scholar]
- Rodriguez Chanfrau, J.E. Evaluation of the influence of microwaves radiation on a biomaterial composed of three phases of calcium phosphates. Biointerface Res. Appl. Chem. 2020, 5141–5144. [Google Scholar]
- Henise, J.; Yao, B.; Ashley, G.W.; Santi, D.V. Autoclave sterilization of tetra-polyethylene glycol hydrogel biomaterials with β-eliminative crosslinks. Eng. Rep. 2020, 2, e12091. [Google Scholar] [CrossRef] [Green Version]
- Soares, G.C.; Learmonth, D.A.; Vallejo, M.C.; Davila, S.P.; González, P.; Sousa, R.A.; Oliveira, A.L. Supercritical CO2 technology: The next standard sterilization technique? Mater. Sci. Eng. C 2019, 99, 520–540. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Paneva, D.; Mincheva, R.; Dubois, P.; Rashkov, I.; Maximova, V.; Danchev, D. Polylactide stereocomplex-based electrospun materials possessing surface with antibacterial and hemostatic properties. Biomacromolecules 2010, 11, 151–159. [Google Scholar] [CrossRef]
- Yancheva, E.; Paneva, D.; Maximova, V.; Mespouille, L.; Dubois, P.; Manolova, N.; Rashkov, I. Polyelectrolyte complexes between (cross-linked) N-carboxyethylchitosan and (quaternized) poly [2-(dimethylamino) ethyl methacrylate]: Preparation, characterization, and antibacterial properties. Biomacromolecules 2007, 8, 976–984. [Google Scholar] [CrossRef]
- Yancheva, E.; Paneva, D.; Danchev, D.; Mespouille, L.; Dubois, P.; Manolova, N.; Rashkov, I. Polyelectrolyte complexes based on (quaternized) poly [(2-dimethylamino) ethyl methacrylate]: Behavior in contact with blood. Macromol. Biosci. 2007, 7, 940–954. [Google Scholar] [CrossRef]
- Huang, J.; Murata, H.; Koepsel, R.R.; Russell, A.J.; Matyjaszewski, K.J.B. Antibacterial polypropylene via surface-initiated atom transfer radical polymerization. Biomacromolecules 2007, 8, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Ajiro, H.; Ito, S.; Kan, K.; Akashi, M. Catechin-modified polylactide stereocomplex at chain End improved antibiobacterial property. Macromol. Biosci. 2016, 16, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fukushima, K.; Coady, D.J.; Engler, A.C.; Liu, S.; Huang, Y.; Cho, J.S.; Guo, Y.; Miller, L.S.; Tan, J.P. Broad-spectrum antimicrobial and biofilm-disrupting hydrogels: Stereocomplex-driven supramolecular assemblies. Angew. Chem. 2013, 125, 702–706. [Google Scholar] [CrossRef]
- Mei, L.; Ren, Y.; Gu, Y.; Li, X.; Wang, C.; Du, Y.; Fan, R.; Gao, X.; Chen, H.; Tong, A. Strengthened and thermally resistant poly (lactic acid)-based composite nanofibers prepared via easy stereocomplexation with antibacterial effects. ACS Appl. Mater. Interfaces 2018, 10, 42992–43002. [Google Scholar] [CrossRef]
- Ren, Y.; Mei, L.; Gu, Y.; Zhao, N.; Wang, Y.; Fan, R.; Tong, A.; Chen, H.; Yang, H.; Han, B. Stereocomplex crystallite-based eco-friendly nanofiber membranes for removal of Cr (VI) and antibacterial effects. ACS Sustain. Chem. Eng. 2019, 7, 16072–16083. [Google Scholar] [CrossRef]



















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, S.H.; Im, D.H.; Park, S.J.; Chung, J.J.; Jung, Y.; Kim, S.H. Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review. Molecules 2021, 26, 2846. https://doi.org/10.3390/molecules26102846
Im SH, Im DH, Park SJ, Chung JJ, Jung Y, Kim SH. Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review. Molecules. 2021; 26(10):2846. https://doi.org/10.3390/molecules26102846
Chicago/Turabian StyleIm, Seung Hyuk, Dam Hyeok Im, Su Jeong Park, Justin Jihong Chung, Youngmee Jung, and Soo Hyun Kim. 2021. "Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review" Molecules 26, no. 10: 2846. https://doi.org/10.3390/molecules26102846
APA StyleIm, S. H., Im, D. H., Park, S. J., Chung, J. J., Jung, Y., & Kim, S. H. (2021). Stereocomplex Polylactide for Drug Delivery and Biomedical Applications: A Review. Molecules, 26(10), 2846. https://doi.org/10.3390/molecules26102846