Quantification of Volatile Aldehydes Deriving from In Vitro Lipid Peroxidation in the Breath of Ventilated Patients
Abstract
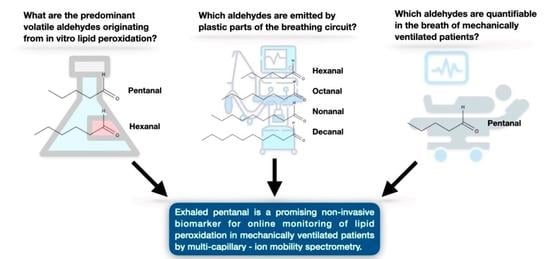
:1. Introduction
2. Results
2.1. Calibration
2.2. Volatile Aldehydes Originating from In Vitro Lipid Peroxidation
2.3. Volatile Aldehydes Emitted By Plastic Parts of the Breathing Circuit
2.4. Volatile Aldehydes in the Breath of Ventilated Patients
3. Discussion
4. Materials and Methods
4.1. Calibration
4.2. Volatile Aldehydes Originating from In Vitro Lipid Peroxidation
4.3. Volatile Aldehydes Emitted by Plastic Parts of the Breathing Circuit
4.4. Volatile Aldehydes in the Breath of Ventilated Patients
4.4.1. Ethics
4.4.2. Inclusion and Exclusion Criteria
4.4.3. Measurements
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niki, E. Biomarkers of lipid peroxidation in clinical material. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 809–817. [Google Scholar] [CrossRef]
- Schallschmidt, K.; Becker, R.; Jung, C.; Bremser, W.; Walles, T.; Neudecker, J.; Leschber, G.; Frese, S.; Nehls, I. Comparison of volatile organic compounds from lung cancer patients and healthy controls—Challenges and limitations of an observational study. J. Breath Res. 2016. [Google Scholar] [CrossRef]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, A.; Kowalkowski, T.; Trawińska, E.; Buszewski, B. The application of statistical methods using VOCs to identify patients with lung cancer. J. Breath Res. 2011, 5, 046008. [Google Scholar] [CrossRef] [PubMed]
- Corradi, M.; Rubinstein, I.; Andreoli, R.; Manini, P.; Caglieri, A.; Poli, D.; Alinovi, R.; Mutti, A. Aldehydes in Exhaled Breath Condensate of Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Grassin-Delyle, S.; Roquencourt, C.; Moine, P.; Saffroy, G.; Carn, S.; Heming, N.; Fleuriet, J.; Salvator, H.; Naline, E.; Couderc, L.J.; et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study. EBioMedicine 2021, 63, 103154. [Google Scholar] [CrossRef]
- Ruszkiewicz, D.M.; Sanders, D.; O’Brien, R.; Hempel, F.; Reed, M.J.; Riepe, A.C.; Bailie, K.; Brodrick, E.; Darnley, K.; Ellerkmann, R.; et al. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry—A feasibility study. EClinicalMedicine 2020, 29–30, 100609. [Google Scholar] [CrossRef]
- Lichtenstern, C.; Hofer, S.; Möllers, A.; Snyder-Ramos, S.; Spies-Martin, D.; Martin, E.; Schmidt, J.; Motsch, J.; Bardenheuer, H.J.; Weigand, M.A. Lipid peroxidation in acute respiratory distress syndrome and liver failure. J. Surg. Res. 2011, 168, 243–252. [Google Scholar] [CrossRef]
- Weigand, M.A.; Snyder-Ramos, S.A.; Möllers, A.G.; Bauer, J.; Hansen, D.; Kochen, W.; Martin, E.; Motsch, J. Inhaled nitric oxide does not enhance lipid peroxidation in patients with acute respiratory distress syndrome. Crit. Care Med. 2000, 28, 3429–3435. [Google Scholar] [CrossRef]
- Müller-Wirtz, L.M.; Kiefer, D.; Maurer, F.; Floss, M.A.; Doneit, J.; Hüppe, T.; Shopova, T.; Wolf, B.; Sessler, D.I.; Volk, T.; et al. Volutrauma Increases Exhaled Pentanal in Rats: A Potential Breath Biomarker for Ventilator-Induced Lung Injury. Anesth. Analg. 2021. Published Ahead-of-Print. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, K.; Sano, M.; Fujita, M.; Tomita, I. Production of Aliphatic Aldehydes on Peroxidation of Various Types of Lipids. Chem. Pharm. Bull. 1991, 39, 1788–1791. [Google Scholar] [CrossRef] [Green Version]
- Reinheckel, T.; Noack, H.; Lorenz, S.; Wiswedel, I.; Augustin, W. Comparison of protein oxidation and aldehyde formation during oxidative stress in isolated mitochondria. Free Radic. Res. 1998, 29, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Hu, M.-L.; Tappel, A.L. Rapid headspace gas chromatography of hexanal as a measure of lipid peroxidation in biological samples. Lipids 1989, 24, 976–981. [Google Scholar] [CrossRef]
- Frankel, E.N.; Tappel, A.L. Headspace gas chromatography of volatile lipid peroxidation products from human red blood cell membranes. Lipids 1991, 26, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Shestivska, V.; Olšinová, M.; Sovová, K.; Kubišta, J.; Smith, D.; Cebecauer, M.; Španěl, P. Evaluation of lipid peroxidation by the analysis of volatile aldehydes in the headspace of synthetic membranes using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 1617–1628. [Google Scholar] [CrossRef]
- Rebeyrolle-Bernard, P.; Etiévant, P. Volatile compounds extracted from polypropylene sheets by hot water: Influence of the temperature of sheets injection. J. Appl. Polym. Sci. 1993, 49, 1159–1164. [Google Scholar] [CrossRef]
- Hüppe, T.; Lorenz, D.; Wachowiak, M.; Maurer, F.; Meiser, A.; Groesdonk, H.; Fink, T.; Sessler, D.I.; Kreuer, S. Volatile organic compounds in ventilated critical care patients: A systematic evaluation of cofactors. BMC Pulm. Med. 2017, 17, 116. [Google Scholar] [CrossRef]
- Hüppe, T.; Klasen, R.; Maurer, F.; Meiser, A.; Groesdonk, H.-V.; Sessler, D.I.; Fink, T.; Kreuer, S. Volatile Organic Compounds in Patients With Acute Kidney Injury and Changes During Dialysis. Crit. Care Med. 2019, 47, 239–246. [Google Scholar] [CrossRef]
- Wirtz, L.M.; Kreuer, S.; Volk, T.; Hüppe, T. Moderne Atemgasanalysen. Med. Klin.-Intensivmed. Notf. 2019, 114, 655–660. [Google Scholar] [CrossRef]
- Perl, T.; Carstens, E.; Hirn, A.; Quintel, M.; Vautz, W.; Nolte, J.; Jünger, M. Determination of serum propofol concentrations by breath analysis using ion mobility spectrometry. Br. J. Anaesth. 2009, 103, 822–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuder, A.-E.; Buchinger, H.; Kreuer, S.; Volk, T.; Maddula, S.; Baumbach, J.I. Characterization of propofol in human breath of patients undergoing anesthesia. Int. J. Ion Mobil. Spectrom. 2011, 14, 167–175. [Google Scholar] [CrossRef]
- Müller-Wirtz, L.M.; Maurer, F.; Brausch, T.; Kiefer, D.; Floss, M.; Doneit, J.; Volk, T.; Sessler, D.I.; Fink, T.; Lehr, T.; et al. Exhaled Propofol Concentrations Correlate With Plasma and Brain Tissue Concentrations in Rats. Anesth. Analg. 2021, 132, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kumar, S.; Hanna, G.B. Investigation of C3-C10 aldehydes in the exhaled breath of healthy subjects using selected ion flow tube-mass spectrometry (SIFT-MS). J. Breath Res. 2014, 8, 037104. [Google Scholar] [CrossRef]
- McCartney, M.M.; Thompson, C.J.; Klein, L.R.; Ngo, J.H.; Seibel, J.D.; Fabia, F.; Simms, L.A.; Borras, E.; Young, B.S.; Lara, J.; et al. Breath carbonyl levels in a human population of seven hundred participants. J. Breath Res. 2020, 14, 046005. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. C5 to C10 Aliphatic Aldehydes. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 28 February 2021).
- National Center for Biotechnology Information. PubChem Database. Hexanal, CID=6184. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hexanal (accessed on 20 January 2020).
- National Center for Biotechnology Information. PubChem Database. Pentanal, CID=8063. Available online: https://pubchem.ncbi.nlm.nih.gov/compound (accessed on 20 January 2020).
- Bravo, A.; Hotchkiss, J.H. Identification of volatile compounds resulting from the thermal oxidation of polyethylene. J. Appl. Polym. Sci. 1993, 47, 1741–1748. [Google Scholar] [CrossRef]
- Panseri, S.; Chiesa, L.M.; Zecconi, A.; Soncini, G.; De Noni, I. Determination of Volatile Organic Compounds (VOCs) from wrapping films and wrapped PDO Italian cheeses by using HS-SPME and GC/MS. Molecules 2014, 19, 8707–8724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.T.; Yang, C.Y.; Miao, L.J.; Zhang, S.F.; Han, X.P.; Ren, S.E.; Sun, X.Q.; Cao, Y.N. Effects of mechanical ventilation with different tidal volume on oxidative stress and antioxidant in lung. J. Anesth. 2015, 29, 346–351. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of potential breath biomarkers for the early diagnosis of breast cancer using gas chromatography–mass spectrometry. Clin. Chim. Acta 2014, 436, 59–67. [Google Scholar] [CrossRef]
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Mackenzie, H.A.; Veselkov, K.A.; Hoare, J.M.; Lovat, L.B.; Španěl, P.; Smith, D.; Hanna, G.B. Mass Spectrometric Analysis of Exhaled Breath for the Identification of Volatile Organic Compound Biomarkers in Esophageal and Gastric Adenocarcinoma. Ann. Surg. 2015, 262, 981–990. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, B.; Liu, N.; Ge, H.; Hong, Y. Mechanical power normalized to predicted body weight as a predictor of mortality in patients with acute respiratory distress syndrome. Intensive Care Med. 2019, 45, 856–864. [Google Scholar] [CrossRef]
- Cressoni, M.; Gotti, M.; Chiurazzi, C.; Massari, D.; Algieri, I.; Amini, M.; Cammaroto, A.; Brioni, M.; Montaruli, C.; Nikolla, K.; et al. Mechanical power and development of ventilator-induced lung injury. Anesthesiology 2016, 124, 1100–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, J.K.; Spittler, K.-H.; Braun, G.; Geiger, K.; Guttmann, J. CO 2 -controlled sampling of alveolar gas in mechanically ventilated patients. J. Appl. Physiol. 2001, 90, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Müller-Wirtz, L.M.; Godsch, C.; Sessler, D.I.; Volk, T.; Kreuer, S.; Hüppe, T. Residual volatile anesthetics after workstation preparation and activated charcoal filtration. Acta Anaesthesiol. Scand. 2020, 64, 759–765. [Google Scholar] [CrossRef]
- Chiumello, D.; Gotti, M.; Guanziroli, M.; Formenti, P.; Umbrello, M.; Pasticci, I.; Mistraletti, G.; Busana, M. Bedside calculation of mechanical power during volume- and pressure-controlled mechanical ventilation. Crit. Care 2020, 24, 417. [Google Scholar] [CrossRef]
- Dixon, W.J. Analysis of Extreme Values. Ann. Math. Stat. 1950, 21, 488–506. [Google Scholar] [CrossRef]
- Zheng, B. Summarizing the goodness of fit of generalized linear models for longitudinal data. Stat. Med. 2000, 19, 1265–1275. [Google Scholar] [CrossRef]



| Material | Detected Aldehydes | Concentration (ppb) |
|---|---|---|
| Endotracheal tube | Octanal | 7.0 ± 1.4 |
| Nonanal | 12.5 ± 0.7 | |
| Decanal | 2.5 ± 0.4 | |
| Humidity and moisture exchanging filter | Nonanal | 0.1 ± 0.4 |
| Decanal | 1.7 ± 0.1 | |
| Breathing bag | Hexanal | 0.5 ± 0.1 |
| Nonanal | 2.0 ± 0.3 | |
| Decanal | 0.7 ± 0.1 | |
| Breathing tubes | Hexanal | 0.2 ± 0.1 |
| Nonanal | 1.4 ± 0.2 | |
| Decanal | 0.6 ± 0.2 | |
| Test lung | Nonanal | unquantifiable traces |
| Decanal | 0.8 ± 0.2 |
| Patient Characteristics | |
| Patients included/screened for eligibility | 12/12 |
| Age (years) | 67 ± 11 |
| Sex (male/female) | 8 (67)/4 (33) |
| Height (cm) | 170 ± 8 |
| Weight (kg) | 69 ± 13 |
| ASA physical status (I/II/III) | 0/6 (50)/6 (50) |
| Malignant tumor | 8 (67) |
| Arterial hypertension | 8 (67) |
| Diabetes mellitus | 6 (50) |
| Mechanical ventilation time (min) | 344 ± 102 |
| Ventilation Parameters | |
| Tidal volume (mL) | 452 ± 82 |
| Respiratory rate (breaths·min−1) | 12 ± 1 |
| Minute volume (L·min−1) | 5.4 ± 1.1 |
| Inspiratory pressure (mbar) | 15.3 ± 2.1 |
| Positive end expiratory pressure (mbar) | 5.1 ± 0.5 |
| Mechanical power (J·min−1) | 8.3 ± 2.6 |
| Parameter | Regression Coefficient | 95% Confidence Interval | R² | p |
|---|---|---|---|---|
| Tidal volume (mL) | 0.01 | 0.003–0.018 | 0.02 | 0.004 |
| Minute volume (L·min−1) | 2.0 | 0.6–3.3 | 0.05 | 0.004 |
| Inspiratory pressure (mbar) | 0.2 | −0.3–0.6 | 0.04 | 0.463 |
| Mechanical power (J·min−1) | 0.7 | 0.3–1.1 | 0.11 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller-Wirtz, L.M.; Kiefer, D.; Ruffing, S.; Brausch, T.; Hüppe, T.; Sessler, D.I.; Volk, T.; Fink, T.; Kreuer, S.; Maurer, F. Quantification of Volatile Aldehydes Deriving from In Vitro Lipid Peroxidation in the Breath of Ventilated Patients. Molecules 2021, 26, 3089. https://doi.org/10.3390/molecules26113089
Müller-Wirtz LM, Kiefer D, Ruffing S, Brausch T, Hüppe T, Sessler DI, Volk T, Fink T, Kreuer S, Maurer F. Quantification of Volatile Aldehydes Deriving from In Vitro Lipid Peroxidation in the Breath of Ventilated Patients. Molecules. 2021; 26(11):3089. https://doi.org/10.3390/molecules26113089
Chicago/Turabian StyleMüller-Wirtz, Lukas Martin, Daniel Kiefer, Sven Ruffing, Timo Brausch, Tobias Hüppe, Daniel I. Sessler, Thomas Volk, Tobias Fink, Sascha Kreuer, and Felix Maurer. 2021. "Quantification of Volatile Aldehydes Deriving from In Vitro Lipid Peroxidation in the Breath of Ventilated Patients" Molecules 26, no. 11: 3089. https://doi.org/10.3390/molecules26113089
APA StyleMüller-Wirtz, L. M., Kiefer, D., Ruffing, S., Brausch, T., Hüppe, T., Sessler, D. I., Volk, T., Fink, T., Kreuer, S., & Maurer, F. (2021). Quantification of Volatile Aldehydes Deriving from In Vitro Lipid Peroxidation in the Breath of Ventilated Patients. Molecules, 26(11), 3089. https://doi.org/10.3390/molecules26113089