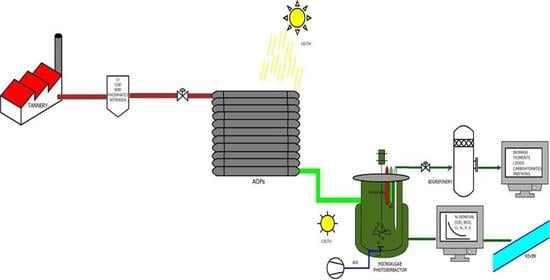
Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater
Abstract
:1. Introduction
2. Pollutant Loads from Tannery Wastewaters
3. Technologies for the Treatment of Tannery Wastewater
3.1. Coagulation
3.2. Electrocoagulation
3.3. Advanced Oxidation Processes (AOPs)
Energy and Cost Considerations in AOPs
3.4. Biotechnological Conversion of Tannery Wastewater
3.4.1. Bacteria
3.4.2. Utilizing Tannery Wastewater in Microbial Fuel Cells
3.4.3. Microalgae
| Strain | Operating Conditions | Parameters | Removal Efficiency | Reference |
|---|---|---|---|---|
| Scenedesmus sp. | V: 1 L; w/w concentration: 20–100%; light intensity: 97.5–182.5 μmol photons m−2 s−1; pH: 7.5; T: 25 °C; time: 25 d. | COD NH3–N PO4–P | COD: 80.33% NH3–N: 85.63% PO4–P: 96.78% | [185] |
| Scenedesmus sp. | V: 3 L; pH: 2−11, T: 25–40 °C; dye concentration: 200−1500 mg L−1; contact time: 540 min. | Absorption of the AB–161 dye pH TOC Total nitrogen (TN) | AB–161: 69.83% TOC: 50.78% TN: 19.80% | [13] |
| Nannochloropsis oculata | V: 0.2 L; light intensity: 75 μmol photons m−2 s−1; photoperiod: 12:12; T: 25 °C; time: 15 d; pH: 7.6. | COD Color Inorganic carbon NH4–N PO4–P Chromium (Cr) TDS | COD: 84% Color: 60% Inorganic carbon: 90% NH4–N: 82% PO4–P: 100% Cr: 97% TDS: 10% | [43] |
| Dunaliella salina | V: 0.25 L; T: 25 °C; pH: 7.5; Cr (10, 20 and 30 mg L−1); culture temperature: 25 ± 2 °C (±1); photoperiod: 24:0; light intensity: 10 Wm−2; time: 120 h. | Cr | Cr: 66.4% | [177] |
| S. quadricauda | V: 1 L; photoperiod: 16:8 h (light/dark); light intensity: 110 μmol photons m−2 s−1; T: 22 °C; pH: 2–7; Cr concentration: 10 mg L−1; time: 8 d. | Cr(VI) | Cr: 98% | [12] |
| Chlorella vulgaris Pseudochlorella pringsheimii | V: 0.3 L; tanning effluents dilution: 10–50%; photoperiod: 24:0 h (light/dark); T: 27 °C; light intensity: 35 μmol photons m−2 s−1. | NH3–N PO4–P COD Cr | NH3–N: 100% PO4–P: 63% COD: 80% Cr: 56% | [43] |
| C. pyrenoidosa Scenedesmus sp. | V: 0.25 L; photoperiod: 12:12 h; T: 27 °C; tannery effluent concentration: 0−10–25–50–75−100%; pH: 7; time: 12 d. | Cr | Cr: 75% | [182] |
| Tetraselmis sp. | V: 0.25 L; T: 24 °C; photoperiod: 24:0 h (light/dark); time 19 d.; tannery effluent concentration: 50–75%. | Total Kjeldahl Nitrogen (TNK) NH3–N PO4–P Chemical oxygen demand (COD) | NH3–N: 99.90%, TKN: 79.36%, PO4–P: 87.82%, COD: 14.26% | [16] |
| C. vulgaris S. acutus | V: 10 L; T: 24 ± 2 °C; pH: 6.3 ± 0.3; time: 8−10 d.; illumination: 4500 ± 50 lux; photoperiod: 16:8 h (light/dark). | Cr | Cr: 88.2% (C. vulgaris), 87.1% (S. acutus) | [184] |
| C. vulgaris | V: 0.1 L; water concentration: 100–70–50–30−10%; T: 28 ± 0.5 °C; fluorescent lights: 150–300 μmol photons m−2 s−1; photoperiod: 10:14 h (light/dark); time: 21 days. | BOD COD NO3–N PO4–P SO4–S Cr | NO3–N: 100% Cr: 91.73% PO4: 99% SO4: 67.4% COD: 94.74% BOD: 95.93% | [42] |
| Scenedesmus sp. C. variabilis C. sorokiniana | V: 0.1 L; T: 25 °C, illumination: 40 μmol photons m−2 s−1; photoperiod: 14:10 h (light/dark); concentration: 25–40–60%. | COD NH4–N PO43−–P | Scenedesmus sp.: COD: 66% and 56% NH4: 47% and 39% PO43−: 70% and 64% C. variabilis: COD: 84% and 80% NH4: 68% and 62% PO43−: 93% and 87% C. sorokiniana: COD: 80% and 74% NH4: 74% and 56% PO43−: 93% and 93% | [2] |
| Scenedesmus sp. | V: 0.5 L; wastewater concentration: 20–50−100%; photoperiod: 16:8 h (light/dark); T: 24 °C; time: 15 d. The 100% concentration was used for nutrient removal experiments. | Cr(VI) NO2–N NO3–N PO4–P SO4–S DBO | Cr+6: 98% NO2: 95% NO3: 90% PO4: 99% SO4: 92% BOD5: 88% | [170] |
| Microalgae consortium. Dominant microalgae: Tetraselmis sp. | V: 0.25 L; wastewater concentration: 50R50S and 75R25S; photoperiod: 12:12 h (light/dark); air flow: 1 L min−1; time: 20 days. | PO4–P Total nitrogen (TN) NH3–N COD TOC BOD | 50R50S: PO4: 97.6%, TN: 71.7%, NH3: 100%, COD: 50.4%, TOC: 20%, BOD5: 16.8% 75R25S:PO4: 95.5%, TN: 58.8%, NH3: 100%, COD: 56.7%, TOC: 31.3%, BOD5: 20.7% | [7] |
| Scenedesmus sp. | V: 0.15 L; tannery wastewater dilutions: 10%, 25%, 50%, 75% and 100%; T: 27 ± 2 °C; illumination: 4000 lux; photoperiod: 16:8 h (dark/light); time: 12 d. | Cr Cu Pb Zn NO3 PO4 | Cr: 81.2–96% Cu: 73.2–98% Pb: 75–98% Zn: 65–98% NO3 > 44.3% PO4 > 95% | [30] |
| C. vulgaris | V: 0.25 L; T: 26 ± 2 °C; illumination: 5000 lux; wastewater concentration: 100%; pH: 7.1; time: 15 d. | NO3–N NH4–N PO4–P COD | NH4: 55% NO3: 85.6% PO4: 60.5% COD: 43.4% | [186] |
| Chlorella sp. | V: 0.3 L; wastewater tannery concentration: 50−100%; time: 12 d; T: 27.5 °C; illumination: 4000 lux; photoperiod: 12:12 h (fluorescent lamps). | Cr Cu Pb Zn | 50% dilution: Cr: 73.1%, 45.7% Cu: 90.4%, 78.1% Pb: 92.1%, 52.2% Zn: 81.2%, 44.6% 100% wastewater: Cr: 45.7% Cu: 78.1% Pb: 52.2% Zn: 44.6% | [186] |
| Chlorella sp. Phormidium sp. | V: 15 L; tannery wastewater: 100%; time: 20 d; T: 28 °C; light intensity: 225 μmol photons m−2 s−1; photoperiod: 12:12 h | BOD COD TN Total phosphorus (TP) Cr TDS | BOD: 93.4% COD: 96.6 ± 11.1% TN: 91.16% TP: 88% Cr: 94.45% TDS: 58.28% | [173] |
4. Present and Future Prospects
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AOPs | advanced oxidation processes |
| BOD | biological oxygen demand |
| Cr | chromium |
| COD | chemical oxygen demand |
| EC | electrochemical |
| EF | electro-Fenton |
| EEO | electrical energy per order |
| EEM | electrical energy per mass |
| H2O2 | peroxide hydrogen |
| LP-UV | low-pressure ultraviolet lamp |
| MFC | microbial fuel cell |
| MP-UV | medium-pressure ultraviolet lamp |
| MLVSS | suspended solids of mixed liquor |
| O3 | ozone |
| TDS | total dissolved solids |
| TKN | total Kjeldahl nitrogen |
| TOC | total organic carbon |
| TSS | total suspended solids |
| US | ultrasound |
| UV | ultraviolet |
References
- Gallego-Molina, A.; Mendoza-Roca, J.A.; Aguado, D.; Galiana-Aleixandre, M.V. Reducing Pollution from the Deliming–Bating Operation in a Tannery. Wastewater Reuse by Microfiltration Membranes. Chem. Eng. Res. Des. 2013, 91, 369–376. [Google Scholar] [CrossRef]
- Nagi, M.; He, M.; Li, D.; Gebreluel, T.; Cheng, B.; Wang, C. Utilization of Tannery Wastewater for Biofuel Production: New Insights on Microalgae Growth and Biomass Production. Sci. Rep. 2020, 10, 1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAOSTAT. Crops and Livestock Products. Available online: http://www.fao.org/faostat/en/#data/TP (accessed on 22 March 2021).
- The Confederation of National Associations of Tanners and Dressers of the European Community (COTANCE). Available online: https://euroleather.com (accessed on 22 March 2021).
- Martinez, B.S.Y.; Romero, C.J.A. Revisión Del Estado Actual De La Industria De Las Curtiembres En Sus Procesos Y Productos: Un análisis De Su Competitividad. Rev. Fac. Cien. Econ. 2017, 26, 113–124. [Google Scholar]
- Lofrano, G.; Belgiorno, V.; Gallo, M.; Raimo, A.; Meriç, S. Toxicity reduction in leather tanning wastewater by improved coagulation flocculation process. Glob. NEST J. 2006, 8, 151–158. [Google Scholar] [CrossRef]
- Pena, A.C.; Trierweiler, L.F.; Gutterres, M. Influence of photoperiod on biomass production and removal of nutrients from tannery effluents with microalgae consortium. In Proceedings of the XXXV IULTCS Congress 2019; Tegtmeyer, D., Meyer, M., Eds.; Association for Tannery Chemistry and Technology: Dresden, Germany, 2019; Volume 4, p. 19. [Google Scholar]
- Ouaissa, Y.A.; Chabani, M.; Amrane, A.; Bensmaili, A. Integration of Electro Coagulation and Adsorption for the Treatment of Tannery Wastewater–The Case of an Algerian Factory, Rouiba. Procedia Eng. 2012, 33, 98–101. [Google Scholar] [CrossRef]
- Hansen, É.; Monteiro de Aquim, P.; Hansen, A.W.; Cardoso, J.K.; Ziulkoski, A.L.; Gutterres, M. Impact of Post-Tanning Chemicals on the Pollution Load of Tannery Wastewater. J. Environ. Manag. 2020, 269, 110787. [Google Scholar] [CrossRef]
- Aravindhan, R.; Madhan, B.; Rao, J.R.; Nair, B.U.; Ramasami, T. Bioaccumulation of Chromium from Tannery Wastewater: An Approach for Chrome Recovery and Reuse. Environ. Sci. Technol. 2004, 38, 300–306. [Google Scholar] [CrossRef]
- Chhikara, S.; Hooda, A.; Rana, L.; Dhankhar, R. Chromium (VI) Biosorption by Immobilized Aspergillus niger in Continuous Flow System with Special Reference to FTIR Analysis. J. Environ. Biol. 2010, 31, 561–566. Available online: http://jeb.co.in/journal_issues/201009_sep10/paper_03.pdf (accessed on 22 March 2020). [PubMed]
- Daneshvar, E.; Zarrinmehr, M.J.; Kousha, M.; Hashtjin, A.M.; Saratale, G.D.; Maiti, A.; Vithanage, M.; Bhatnagar, A. Hexavalent Chromium Removal from Water by Microalgal-Based Materials: Adsorption, Desorption and Recovery Studies. Bioresour. Technol. 2019, 293, 122064. [Google Scholar] [CrossRef]
- da Fontoura, J.T.; Rolim, G.S.; Farenzena, M.; Gutterres, M. Influence of Light Intensity and Tannery Wastewater Concentration on Biomass Production and Nutrient Removal by Microalgae Scenedesmus sp. Process Safe Environ. Prot. 2017, 111, 355–362. [Google Scholar] [CrossRef]
- Mohd Udaiyappan, A.F.; Abu Hasan, H.; Takriff, M.S.; Sheikh Abdullah, S.R. A Review of the Potentials, Challenges and Current Status of Microalgae Biomass Applications in Industrial Wastewater Treatment. J. Water Process Eng. 2017, 20, 8–21. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Pena, A.d.C.C.; Bertoldi, C.F.; da Fontoura, J.T.; Trierweiler, L.F.; Gutterres, M. Consortium of Microalgae for Tannery Effluent Treatment. Braz. Arch. Biol. Technol. 2019, 62. [Google Scholar] [CrossRef]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef] [Green Version]
- Guiza-Franco, L.; Orozco-Rojas, L.G.; Sánchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Production of Chlorella vulgaris Biomass on Uv-Treated Wastewater as an Alternative for Environmental Sustainability on High-Mountain Fisheries. Chem. Eng. Trans. 2018, 64, 517–522. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.B.; Urbina-Suarez, N.A.; Zuorro, A.; Barajas-Solano, A.F.; Kafarov, V. Fisheries Wastewater as a Sustainable Media for the Production of Algae-Based Products. Chem. Eng. Trans. 2019, 76, 1339–1344. [Google Scholar] [CrossRef]
- Estévez-Landazábal, L.L.; Barajas-Solano, A.F.; Barajas-Ferreira, C.; Kafarov, V. Improvement of lipid productivity on Chlorella vulgaris using waste glycerol and sodium acetate. CTF Cienc. Tecnol. Futuro 2013, 5, 113–126. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0122-53832013000100009 (accessed on 22 March 2021).
- Yen, H.-W.; Chen, P.-W.; Hsu, C.-Y.; Lee, L. The Use of Autotrophic Chlorella vulgaris in Chromium (VI) Reduction under Different Reduction Conditions. J. Taiwan Inst. Chem. Eng. 2017, 74, 1–6. [Google Scholar] [CrossRef]
- Garcia-Martinez, B.; Ayala-Torres, E.; Reyes-Gomez, O.; Zuorro, A.; Barajas-Solano, A.; Barajas-Ferreira, C. Evaluation of a Two-Phase Extraction System of Carbohydrates and Proteins from Chlorella vulgaris Utex 1803. Chem. Eng. Trans. 2016, 49, 355–360. [Google Scholar] [CrossRef]
- Barajas-Solano, A.F.; Guzmán-Monsalve, A.; Kafarov, V. Effect of Carbon-Nitrogen Ratio for the Biomass Production, Hydrocarbons and Lipids on Botryoccus braunii UIS 003. Chem. Eng. Trans. 2016, 49, 247–252. [Google Scholar] [CrossRef]
- Cuéllar-García, D.J.; Rangel-Basto, Y.A.; Urbina-Suarez, N.A.; Barajas-Solano, A.F.; Muñoz-Peñaloza, Y.A. Lipids production from Scenedesmus obliquus through carbon/nitrogen ratio optimization. J. Phys. Conf. Ser. 2019, 1388, 012043. [Google Scholar] [CrossRef]
- Cuéllar-García, D.J.; Rangel-Basto, Y.A.; Barajas-Solano, A.F.; Muñoz-Peñaloza, Y.A.; Urbina-Suarez, N.A. Towards the production of microalgae biofuels: The effect of the culture medium on lipid deposition. BioTechnologia 2019, 100, 273–278. [Google Scholar] [CrossRef]
- Zuorro, A.; Leal-Jerez, A.G.; Morales-Rivas, L.K.; Mogollón-Londoño, S.O.; Sanchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Solano, A.F. Enhancement of Phycobiliprotein Accumulation in Thermotolerant Oscillatoria sp. through Media Optimization. ACS Omega 2021, 6, 10527–10536. [Google Scholar] [CrossRef]
- Rangel-Basto, Y.A.; García-Ochoa, I.E.; Suarez-Gelvez, J.H.; Zuorro, A.; Barajas-Solano, A.F.; Urbina-Suarez, N.A. The Effect of Temperature and Enzyme Concentration in the Transesterification Process of Synthetic Microalgae Oil. Chem. Eng. Trans. 2018, 64, 331–336. [Google Scholar] [CrossRef]
- Barajas-Solano, A.F.; Gonzalez-Delgado, A.D.; Kafarov, V. Effect of Thermal Pre-Treatment on Fermentable Sugar Production of Chlorella vulgaris. Chem. Eng. Trans. 2014, 37, 655–660. [Google Scholar] [CrossRef]
- Zuorro, A.; García-Martínez, J.B.; Barajas-Solano, A.F. The Application of Catalytic Processes on the Production of Algae-Based Biofuels: A Review. Catalysts 2021, 11, 22. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Selvaraju, M.; Unnikannan, P.; Sruthi, P. Phycoremediation of Tannery Wastewater Using Microalgae Scenedesmus Species. Int. J. Phytoremed. 2015, 17, 907–916. [Google Scholar] [CrossRef]
- Suárez Escobar, A.F.; García Ubaque, C.A.; Vaca Bohórquez, M.L. Identificación y evaluación de la contaminación del agua por curtiembres en el municipio de Villapinzón. Tecnura 2012, 16, 185–193. [Google Scholar] [CrossRef]
- Carreño, S.U.F. Diseño y evaluación de un biosistema de tratamiento a escala piloto de aguas de curtiembres a través de la Eichhornia crassipes. Rev. Colomb. Biotecnol. 2016, 18, 74–81. [Google Scholar] [CrossRef]
- Quintero Salamanca, G.P.; Quijano Parra, A.; Melendez Gelvez, I. Efecto genotoxico del agua residual de la curtiembre San Faustino—Norte de Santander—Colombia. Rev. Colomb. Tecnol. Av. 2018, 2, 8–16. [Google Scholar] [CrossRef]
- Schilling, K.; Bletterie, U.; Kroiss, H.; Zessner, M. Adapting the Austrian Edict on Wastewater Emissions for Tanneries as Consequence of Foam Formation on Surface Waters. Environ. Sci. Policy 2012, 23, 68–73. [Google Scholar] [CrossRef]
- Genawi, N.M.; Ibrahim, M.H.; El-Naas, M.H.; Alshaik, A.E. Chromium Removal from Tannery Wastewater by Electrocoagulation: Optimization and Sludge Characterization. Water 2020, 12, 1374. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Zouboulis, A.I. Application of Composite Pre-Polymerized Coagulants for the Treatment of High-Strength Industrial Wastewaters. Water 2020, 12, 1258. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Mishra, S. Hexavalent Chromium Reduction Potential of Cellulosimicrobium sp. Isolated from Common Effluent Treatment Plant of Tannery Industries. Ecotoxicol. Environ. Saf. 2018, 147, 102–109. [Google Scholar] [CrossRef]
- Palanisamy, D.; Chockalingam, L.R.; Murugan, D. Microbial Fuel Cell for Effluent Treatment and Sustainable Power Generation. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–13. [Google Scholar] [CrossRef]
- Tamil Selvan, S.; Velramar, B.; Ramamurthy, D.; Balasundaram, S.; Sivamani, K. Pilot Scale Wastewater Treatment, CO2 Sequestration and Lipid Production Using Microalga, Neochloris aquatica RDS02. Int. J. Phytoremed. 2020, 22, 1462–1479. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Mohammed, A.K.; Shuaib, D.T. Potential of Using Kaolin as a Natural Adsorbent for the Removal of Pollutants from Tannery Wastewater. Heliyon 2019, 5, e02923. [Google Scholar] [CrossRef]
- Yadav, A.; Raj, A.; Purchase, D.; Ferreira, L.F.R.; Saratale, G.D.; Bharagava, R.N. Phytotoxicity, Cytotoxicity and Genotoxicity Evaluation of Organic and Inorganic Pollutants Rich Tannery Wastewater from a Common Effluent Treatment Plant (CETP) in Unnao District, India Using Vigna radiata and Allium cepa. Chemosphere 2019, 224, 324–332. [Google Scholar] [CrossRef]
- Das, C.; Naseera, K.; Ram, A.; Meena, R.M.; Ramaiah, N. Bioremediation of Tannery Wastewater by a Salt-Tolerant Strain of Chlorella Vulgaris. J. Appl. Phycol. 2017, 29, 235–243. [Google Scholar] [CrossRef]
- Saranya, D.; Shanthakumar, S. An Integrated Approach for Tannery Effluent Treatment with Ozonation and Phycoremediation: A Feasibility Study. Environ. Res. 2020, 183, 109163. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Serrano, A.; López-Alejo, J.E.; Hernández-Cortázar, M.A.; Elizalde, I. Removing Contaminants from Tannery Wastewater by Chemical Precipitation Using CaO and Ca(OH)2. Chin. J. Chem. Eng. 2020, 28, 1107–1111. [Google Scholar] [CrossRef]
- Khanh, T.K.; Jyh, L.H.; Quyet, V.T.; Tam, M.M.; Anh, P.T.; Kiefer, R. Hydrogen Production from the Tannery Wastewater Treatment by Using Agriculture Supports Membrane/Adsorbents Electrochemical System. Int. J. Hydrogen Energy 2020, 45, 3699–3711. [Google Scholar] [CrossRef]
- Boujelben, R.; Ellouze, M.; Sayadi, S. Detoxification Assays of Tunisian Tannery Wastewater under Nonsterile Conditions Using the Filamentous Fungus Aspergillus niger. BioMed Res. Int. 2019, 2019, 9020178. [Google Scholar] [CrossRef] [Green Version]
- Goswami, S.; Mazumder, D. Treatment of Chrome Tannery Wastewater by Biological Process–A Mini Review. Int. J. Environ. Ecol. Eng. 2013, 7, 798–804. [Google Scholar]
- El Mouhri, G.; Merzouki, M.; Belhassan, H.; Miyah, Y.; Amakdouf, H.; Elmountassir, R.; Lahrichi, A. Continuous Adsorption Modeling and Fixed Bed Column Studies: Adsorption of Tannery Wastewater Pollutants Using Beach Sand. J. Chem. 2020, 2020, 7613484. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Ascón, E.; Marrufo-Saldaña, L.; Neyra-Ascón, W. Reduction of Total Chromium Levels from Raw Tannery Wastewater via Electrocoagulation Using Response Surface Methodology. J. Ecol. Eng. 2019, 20, 217–224. [Google Scholar] [CrossRef]
- Korpe, S.; Bethi, B.; Sonawane, S.H.; Jayakumar, K.V. Tannery Wastewater Treatment by Cavitation Combined with Advanced Oxidation Process (AOP). Ultrason. Sonochem. 2019, 59, 104723. [Google Scholar] [CrossRef] [PubMed]
- Alemu, T.; Mekonnen, A.; Leta, S. Integrated Tannery Wastewater Treatment for Effluent Reuse for Irrigation: Encouraging Water Efficiency and Sustainable Development in Developing Countries. J. Water Process Eng. 2019, 30, 100514. [Google Scholar] [CrossRef]
- Caliari, P.C.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Tannery Wastewater: Organic Load and Sulfide Removal Dynamics by Electrochemical Oxidation at Different Anode Materials. Environ. Technol. Innov. 2019, 14, 100345. [Google Scholar] [CrossRef]
- Hashem, M.A.; Momen, M.A.; Hasan, M.; Nur-A-Tomal, M.S.; Sheikh, M.H.R. Chromium Removal from Tannery Wastewater Using Syzygium Cumini Bark Adsorbent. Int. J. Environ. Sci. Technol. 2019, 16, 1395–1404. [Google Scholar] [CrossRef]
- Ashraf, S.; Naveed, M.; Afzal, M.; Ashraf, S.; Rehman, K.; Hussain, A.; Zahir, Z.A. Bioremediation of Tannery Effluent by Cr- and Salt-Tolerant Bacterial Strains. Environ. Monit. Assess. 2018, 190, 716. [Google Scholar] [CrossRef]
- Kozik, V.; Barbusinski, K.; Thomas, M.; Sroda, A.; Jampilek, J.; Sochanik, A.; Smolinski, A.; Bak, A. Taguchi Method and Response Surface Methodology in the Treatment of Highly Contaminated Tannery Wastewater Using Commercial Potassium Ferrate. Materials 2019, 12, 3784. [Google Scholar] [CrossRef] [Green Version]
- Meenachi, S.; Kandasamy, S. Pre-Treatment of Tannery Chrome Wastewater by Green Synthesised Iron Oxide Nanocatalyst. Int. J. Environ. Anal. Chem. 2019, 1–13. [Google Scholar] [CrossRef]
- Ullah, R.; Ahmad, W.; Ahmad, I.; Khan, M.; Iqbal Khattak, M.; Hussain, F. Adsorption and Recovery of Hexavalent Chromium from Tannery Wastewater over Magnetic Max Phase Composite. Sep. Sci. Technol. 2021, 56, 439–452. [Google Scholar] [CrossRef]
- Le, L.T. Tannery Wastewater Treatment after Activated Sludge Pre-Treatment Using Electro-Oxidation on Inactive Anodes. Clean Technol. Environ. Policy 2020, 22, 1701–1713. [Google Scholar] [CrossRef]
- Pal, M.; Malhotra, M.; Mandal, M.K.; Paine, T.K.; Pal, P. Recycling of Wastewater from Tannery Industry through Membrane-Integrated Hybrid Treatment Using a Novel Graphene Oxide Nanocomposite. J. Water Process Eng. 2020, 36, 101324. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, W.; De Costa, Y.G.; Zhuang, W.-Q.; Yi, S. Assessing Inorganic Components of Cake Layer in A/O Membrane Bioreactor for Physical-Chemical Treated Tannery Effluent. Chemosphere 2020, 250, 126220. [Google Scholar] [CrossRef] [PubMed]
- Zapana, J.S.P.; Arán, D.S.; Bocardo, E.F.; Harguinteguy, C.A. Treatment of Tannery Wastewater in a Pilot Scale Hybrid Constructed Wetland System in Arequipa, Peru. Int. J. Environ. Sci. Technol. 2020, 17, 4419–4430. [Google Scholar] [CrossRef]
- Arukula, D.; Prem, P.; Tanwi, P.; Hariraj, S.; Vijay, L.M.; Brijesh, K.M. Treatment of tannery wastewater using aluminium formate: Influence of the formate over sulphate-based coagulant. Glob. NEST J. 2018, 20, 458–464. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Shekhawat, K.; Gupta, N. Bioreduction of Toxic Hexavalent Chromium by Novel Indigenous Microbe Brevibacillus agri Isolated from Tannery Wastewater. Int. J. Environ. Sci. Technol. 2019, 16, 3549–3556. [Google Scholar] [CrossRef]
- Chaudhary, P.; Beniwal, V.; Kaur, R.; Kumar, R.; Kumar, A.; Chhokar, V. Efficacy of Aspergillus Fumigatus MCC 1175 for Bioremediation of Tannery Wastewater. Clean Soil Air Water 2019, 47, 1900131. [Google Scholar] [CrossRef]
- Belay, A. Impacts of Chromium from Tannery Effluent and Evaluation of Alternative Treatment Options. J. Environ Prot. 2010, 1, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Cotman, M.; Zagorc-Končan, J.; Žgajnar-Gotvajn, A. The Relationship between Composition and Toxicity of Tannery Wastewater. Water Sci. Technol. 2004, 49, 39–46. [Google Scholar] [CrossRef]
- Ashley, B.B.; Puspha, L.E.; Annadurai, G. Physicochemical Characteristics, Isolation and Screening Of Bacteria For Degradation Of Dyes From Tannery Effluents. Res. J. Life Sci. Bioinf. Pharm. Chem. Sci. 2019, 5, 144–158. [Google Scholar] [CrossRef]
- Gutterres, M.; Mella, B. Chromium in Tannery Wastewater. In Heavy Metals in Water: Presence, Removal and Safety; The Royal Society of Chemistry: London, UK, 2015; pp. 315–344. [Google Scholar] [CrossRef]
- Prabhahar, C.; Saleshrani, K.; Tharmaraj, K. Seasonal Variation of Heavy Metals Distribution and Sediments in Palar River in and Around Vaniyambadi Segment, Vellore District, Tamil Nadu, India. Int. J. Pharm. Biol. Sci. Arch. 2012, 3, 112–116. Available online: https://www.ijpba.info/ijpba/index.php/ijpba/article/view/546 (accessed on 22 March 2021).
- Sawalha, H.; Alsharabaty, R.; Sarsour, S.; Al-Jabari, M. Wastewater from Leather Tanning and Processing in Palestine: Characterization and Management Aspects. J. Environ. Manag. 2019, 251, 109596. [Google Scholar] [CrossRef] [PubMed]
- Tamersit, S.; Bouhidel, K.-E.; Zidani, Z. Investigation of Electrodialysis Anti-Fouling Configuration for Desalting and Treating Tannery Unhairing Wastewater: Feasibility of by-Products Recovery and Water Recycling. J. Environ. Manag. 2018, 207, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Quijano Parra, A.; Castillo, T.C.; Meléndez Gélvez, I. Potencial mutagénico y genotóxico de aguas residuales de la curtiembre tasajero en la ciudad de Cúcuta, Norte de Santander, Colombia. Rev. U.D.C.A Actual. Divulg. Científica 2015, 18, 13–20. Available online: http://www.scielo.org.co/scielo.php?pid=S0123-42262015000100003&script=sci_abstract&tlng=es (accessed on 22 March 2021). [CrossRef]
- Meriç, S.; De Nicola, E.; Iaccarino, M.; Gallo, M.; Di Gennaro, A.; Morrone, G.; Warnau, M.; Belgiorno, V.; Pagano, G. Toxicity of Leather Tanning Wastewater Effluents in Sea Urchin Early Development and in Marine Microalgae. Chemosphere 2005, 61, 208–217. [Google Scholar] [CrossRef]
- Lv, W.; Zhao, K.; Ma, S.; Kong, L.; Dang, Z.; Chen, J.; Zhang, Y.; Hu, J. Process of Removing Heavy Metal Ions and Solids Suspended in Micro-Scale Intensified by Hydrocyclone. J. Clean. Prod. 2020, 263, 121533. [Google Scholar] [CrossRef]
- Diaz-Angulo, J.; Porras, J.; Mueses, M.; Torres-Palma, R.A.; Hernandez-Ramirez, A.; Machuca-Martinez, F. Coupling of heterogeneous photocatalysis and photosensitized oxidation for diclofenac degradation: Role of the oxidant species. J. Photochem. Photobiol. A Chem. 2019, 383, 112015. [Google Scholar] [CrossRef]
- Song, Z.; Williams, C.J.; Edyvean, R.G.J. Treatment of Tannery Wastewater by Chemical Coagulation. Desalination 2004, 164, 249–259. [Google Scholar] [CrossRef]
- Achouri, O.; Panico, A.; Bencheikh-Lehocine, M.; Derbal, K.; Pirozzi, F. Effect of Chemical Coagulation Pretreatment on Anaerobic Digestion of Tannery Wastewater. J. Environ. Eng. 2017, 143, 4017039. [Google Scholar] [CrossRef]
- Donneys-Victoria, D.; Bermúdez-Rubio, D.; Torralba-Ramírez, B.; Marriaga-Cabrales, N.; Machuca-Martínez, F. Removal of indigo carmine dye by electrocoagulation using magnesium anodes with polarity change. Environ. Sci. Pollut. Res. 2019, 26, 7164–7176. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ramos, J.A.; Saez, C.; Machuca-Martínez, F.; Rodrigo, M.A. Electro-ozonizers: A new approach for an old problem. Sep. Purif. Technol. 2020, 241. [Google Scholar] [CrossRef]
- Mayta, R.; Mayta, J. Remoción de Cromo y Demanda Química de Oxígeno de Aguas Residuales de Curtiembre Por Electrocoagulación. Rev. Soc. Química Perú 2017, 83, 331–340. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1810-634X2017000300008 (accessed on 22 March 2021). [CrossRef]
- Sanchez-Galvis, E.M.; Cardenas-Gutierrez, I.Y.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass. Appl. Sci. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Castellanos-Estupiñan, M.A.; Sánchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Design of an Electroflotation System for the Concentration and Harvesting of Freshwater Microalgae. Chem. Eng. Trans. 2018, 64, 1–6. [Google Scholar] [CrossRef]
- Şengil, İ.A.; Kulaç, S.; Özacar, M. Treatment of Tannery Liming Drum Wastewater by Electrocoagulation. J. Hazard. Mater. 2009, 167, 940–946. [Google Scholar] [CrossRef]
- Deghles, A.; Kurt, U. Treatment of Tannery Wastewater by a Hybrid Electrocoagulation/Electrodialysis Process. Chem. Eng. Process. Process Intensif. 2016, 104, 43–50. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Kaul, S.N.; Neti, R.N. Tannery Wastewater Treatment by Electro-Oxidation Coupled with a Biological Process. J. Appl. Electrochem. 2005, 35, 381–390. [Google Scholar] [CrossRef]
- Mandal, T.; Dasgupta, D.; Mandal, S.; Datta, S. Treatment of Leather Industry Wastewater by Aerobic Biological and Fenton Oxidation Process. J. Hazard. Mater. 2010, 180, 204–211. [Google Scholar] [CrossRef]
- Martín-Domínguez, A.; Rivera-Huerta, M.L.; Pérez-Castrejón, S.; Garrido-Hoyos, S.E.; Villegas-Mendoza, I.E.; Gelover-Santiago, S.L.; Drogui, P.; Buelna, G. Chromium removal from drinking water by redox-assisted coagulation: Chemical versus electrocoagulation. Sep. Purif. Technol. 2018, 200, 266–272. [Google Scholar] [CrossRef] [Green Version]
- da Silva, G.S.; dos Santos, F.A.; Roth, G.; Frankenberg, C.L.C. Electroplating for chromium removal from tannery wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 607–614. [Google Scholar] [CrossRef]
- Protsenko, V.; Danilov, F. Kinetics and mechanism of chromium electrodeposition from formate and oxalate solutions of Cr(III) compounds. Electrochim. Acta 2009, 54, 5666–5672. [Google Scholar] [CrossRef]
- Korshunov, V.N.; Safonov, V.A.; Vykhodtseva, L.N. Structural features of the electrode/solution interface at the reduction of Cr3+(aq) cations on liquid mercury and solid indium electrodes in acidic media. Russ. J. Electrochem. 2008, 44, 255–264. [Google Scholar] [CrossRef]
- Ameta, R.K.; Chohadia, A.; Jain, A.; Punjabi, P.B. Fenton and Photo-Fenton Processes. In Advanced Oxidation Processes for Waste Water Treatment, 1st ed.; Ameta, S.C., Ed.; Academic Press: London, UK, 2018; pp. 49–87. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on Identification of Leather Industry Wastewater Constituents and Its Photocatalytic Treatment. Int. J. Environ. Sci. Technol. 2013, 10, 855–864. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.A.; Hernandez-Ramirez, A.; Colina-Marquez, J.A.; Bustillo-Lecompte, C.F.; Rehmann, L.; Machuca-Martinez, F. Recent developments in the photocatalytic treatment of cyanide wastewater: An approach to remediation and recovery of metals. Processes 2019, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Herazo, R.; Cañaveral-Velásquez, B.; Pérez-Giraldo, K.; Mueses, M.A.; Pinzón-Cárdenas, M.H.; Machuca-Martínez, F. A MATLAB-based application for modeling and simulation of solar Slurry photocatalytic reactors for environmental applications. Water 2020, 12, 2196. [Google Scholar] [CrossRef]
- Vilardi, G.; Ochando-Pulido, J.M.; Stoller, M.; Verdone, N.; Di Palma, L. Fenton Oxidation and Chromium Recovery from Tannery Wastewater by Means of Iron-Based Coated Biomass as Heterogeneous Catalyst in Fixed-Bed Columns. Chem. Eng. J. 2018, 351, 1–11. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S.; Inglese, M.; Nikolau, A.; Belgiorno, V. Fenton Oxidation Treatment of Tannery Wastewater and Tanning Agents: Synthetic Tannin and Nonylphenol Ethoxylate Based Degreasing Agent. Desalin. Water Treat. 2010, 23, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Jessieleena, A.A.; Priyanka, M.; Saravanakumar, M.P. Comparative Study of Fenton, Fe2+/NaOCl and Fe2+/(NH4)2S2O8 on Tannery Sludge Dewaterability, Degradability of Organics and Leachability of Chromium. J. Hazard. Mater. 2021, 402, 123495. [Google Scholar] [CrossRef]
- Borba, F.H.; Pellenz, L.; Bueno, F.; Inticher, J.J.; Braun, L.; Espinoza-Quiñones, F.R.; Trigueros, D.E.G.; de Pauli, A.R.; Módenes, A.N. Pollutant Removal and Biodegradation Assessment of Tannery Effluent Treated by Conventional Fenton Oxidation Process. J. Environ. Chem. Eng. 2018, 6, 7070–7079. [Google Scholar] [CrossRef]
- Twort, A.C.; Ratnayaka, D.D.; Brandt, M.J. (Eds.) Specialised and Advanced Water Treatment Processes. In Water Supply, 5th ed.; Butterworth-Heinemann: London, UK, 2000; pp. 406–408. [Google Scholar] [CrossRef]
- Módenes, A.N.; Espinoza-Quiñones, F.R.; Borba, F.H.; Manenti, D.R. Performance Evaluation of an Integrated Photo-Fenton—Electrocoagulation Process Applied to Pollutant Removal from Tannery Effluent in Batch System. Chem. Eng. J. 2012, 197, 1–9. [Google Scholar] [CrossRef]
- Ikehata, K.; Li, Y. Ozone-Based Processes. In Advanced Oxidation Processes for Waste Water Treatment, 1st ed.; Ameta, S.C., Ed.; Academic Press: London, UK, 2018; pp. 115–143. [Google Scholar] [CrossRef]
- Di Iaconi, C. Biological Treatment and Ozone Oxidation: Integration or Coupling. Bioresour. Technol. 2012, 106, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Radha, K.V.; Sirisha, K. Electrochemical Oxidation Processes. In Advanced Oxidation Processes for Waste Water Treatment, 1st ed.; Ameta, S.C., Ed.; Academic Press: London, UK, 2018; pp. 359–373. [Google Scholar] [CrossRef]
- Houshyar, Z.; Khoshfetrat, A.B.; Fatehifar, E. Influence of Ozonation Process on Characteristics of Pre-Alkalized Tannery Effluents. Chem. Eng. J. 2012, 191, 59–65. [Google Scholar] [CrossRef]
- Sivagami, K.; Sakthivel, K.P.; Nambi, I.M. Advanced Oxidation Processes for the Treatment of Tannery Wastewater. J. Environ. Chem. Eng. 2018, 6, 3656–3663. [Google Scholar] [CrossRef]
- Di Iaconi, C.; Del Moro, G.; De Sanctis, M.; Rossetti, S. A Chemically Enhanced Biological Process for Lowering Operative Costs and Solid Residues of Industrial Recalcitrant Wastewater Treatment. Water Res. 2010, 44, 3635–3644. [Google Scholar] [CrossRef]
- Sundarapandiyan, S.; Chandrasekar, R.; Ramanaiah, B.; Krishnan, S.; Saravanan, P. Electrochemical Oxidation and Reuse of Tannery Saline Wastewater. J. Hazard. Mater. 2010, 180, 197–203. [Google Scholar] [CrossRef]
- Cruz-Rizo, A.; Gutiérrez-Granados, S.; Salazar, R.; Peralta-Hernández, J.M. Application of Electro-Fenton/BDD Process for Treating Tannery Wastewaters with Industrial Dyes. Sep. Purif. Technol. 2017, 172, 296–302. [Google Scholar] [CrossRef]
- Medrano-Rodríguez, F.; Picos-Benítez, A.; Brillas, E.; Bandala, E.R.; Pérez, T.; Peralta-Hernández, J.M. Electrochemical Advanced Oxidation Discoloration and Removal of Three Brown Diazo Dyes Used in the Tannery Industry. J. Electroanal. Chem. 2020, 873, 114360. [Google Scholar] [CrossRef]
- Moradi, M.; Moussavi, G. Enhanced Treatment of Tannery Wastewater Using the Electrocoagulation Process Combined with UVC/VUV Photoreactor: Parametric and Mechanistic Evaluation. Chem. Eng. J. 2019, 358, 1038–1046. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G. Facile Method to Immobilize ZnO Particles on Glass Spheres for the Photocatalytic Treatment of Tannery Wastewater. J. Colloid Interface Sci. 2018, 518, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green Synthesis of TiO2 Nanoparticles Using Leaf Extract of Jatropha curcas L. for Photocatalytic Degradation of Tannery Wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Tien, T.T.; Luu, T.L. Electrooxidation of Tannery Wastewater with Continuous Flow System: Role of Electrode Materials. Environ. Eng. Res. 2020, 25, 324–334. [Google Scholar] [CrossRef]
- Korpe, S.; Rao, P.V. Application of advanced oxidation processes and cavitation techniques for treatment of tannery wastewater—A review. J. Environ. Chem. Eng. 2021, 9, 105234. [Google Scholar] [CrossRef]
- Rahim Pouran, S.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Gurses, F. Photo-Fenton-like and photo-fenton-like oxidation of Procaine Penicillin G formulation effluent. J. Photochem. Photobiol. A Chem. 2004, 3, 165–175. [Google Scholar] [CrossRef]
- Sivakumar, V.; Anna, J.L.; Vijayeeswarri, J.; Swaminathan, G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason. Sonochem. 2009, 16, 782–789. [Google Scholar] [CrossRef]
- Schrank, S.G.; José, H.J.; Moreira, R.F.P.M.; Schröder, H.F. Applicability of fenton and H2O2/UV reactions in the treatment of tannery wastewaters. Chemosphere 2005, 60, 644–655. [Google Scholar] [CrossRef]
- Jiménez, E.; Gilles, M.K.; Ravishankara, A.R. Kinetics of the reactions of the hydroxyl radical with CH3OH and C2H5OH between 235 and 360 K. J. Photochem. Photobiol. A Chem. 2003, 157, 237–245. [Google Scholar] [CrossRef]
- Sun, X.; Liu, J.; Ji, L.; Wang, G.; Zhao, S.; Yoon, J.Y.; Chen, S. A review on hydrodynamic cavitation disinfection: The current state of knowledge. Sci. Total Environ. 2020, 737. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Patil, P.N. Combined treatment technology based on synergism between hydrodynamic cavitation and advanced oxidation processes. Ultrason. Sonochem. 2015, 25, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, E.; Asenath-Smith, E.; Ballard, W.; Moores, L.; Brame, J. Cross-Comparison of Advanced Oxidation Processes for Remediation of Organic Pollutants in Water Treatment Systems. In ERDC Program Element 622720048, “Industrial Operations Pollution Control Guidance”; U.S. Army Corps of Engineers: Washington, DC, USA, 2019. [Google Scholar]
- Report, I.T.; Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar]
- Capodaglio, A.G. Contaminants of emerging concern removal by high-energy oxidation-reduction processes: State of the art. Appl. Sci. 2019, 9, 4562. [Google Scholar] [CrossRef] [Green Version]
- Wardenier, N.; Liu, Z.; Nikiforov, A.; Van Hulle, S.W.H.; Leys, C. Micropollutant elimination by O3, UV and plasma-based AOPs: An evaluation of treatment and energy costs. Chemosphere 2019, 234, 715–724. [Google Scholar] [CrossRef]
- Soares, P.A.; Silva, T.F.C.V.; Ramos Arcy, A.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Assessment of AOPs as a polishing step in the decolourisation of bio-treated textile wastewater: Technical and economic considerations. J. Photochem. Photobiol. A Chem. 2016, 317, 26–38. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef]
- Gomes, A.I.; Soares, T.F.; Silva, T.F.C.V.; Boaventura, R.A.R.; Vilar, V.J.P. Ozone-driven processes for mature urban landfill leachate treatment: Organic matter degradation, biodegradability enhancement and treatment costs for different reactors configuration. Sci. Total Environ. 2020, 724. [Google Scholar] [CrossRef]
- Isarain-chávez, E.; De, C.; Godínez, L.A.; Brillas, E.; Peralta-hernández, J.M. Comparative study of electrochemical water treatment processes for a tannery wastewater effluent. J. Electroanal. Chem. 2014, 713, 62–69. [Google Scholar] [CrossRef]
- Vijayalakshmi, P.; Raju, G.B.; Gnanamani, A. Advanced oxidation and electrooxidation as tertiary treatment techniques to improve the purity of tannery wastewater. Ind. Eng. Chem. Res. 2011, 50, 10194–10200. [Google Scholar] [CrossRef]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and Biological Treatment Technologies for Leather Tannery Chemicals and Wastewaters: A Review. Sci. Total Environ. 2013, 461, 265–281. [Google Scholar] [CrossRef]
- Mpofu, A.B.; Oyekola, O.O.; Welz, P.J. Anaerobic treatment of tannery wastewater in the context of a circular bioeconomy for developing countries. J. Clean. Prod. 2021, 296, 126490. [Google Scholar] [CrossRef]
- GracePavithra, K.; Jaikumar, V.; Kumar, P.S.; SundarRajan, P.S. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Lei, C.; Lin, Y.; Zeng, Y.; Wang, Y.; Yuan, Y.; Shi, B. A Cleaner Deliming Technology with Glycine for Ammonia-Nitrogen Reduction in Leather Manufacture. J. Clean. Prod. 2020, 245, 118900. [Google Scholar] [CrossRef]
- Haydar, S.; Aziz, J.A.; Ahmad, M.S. Biological Treatment of Tannery Wastewater Using Activated Sludge Process. Pak. J. Eng. Appl. Sci. 2007, 1, 61–66. [Google Scholar]
- Fathima, A.; Rao, J.R.; Unni Nair, B. Trivalent Chromium Removal from Tannery Effluent Using Kaolin-Supported Bacterial Biofilm of Bacillus Sp Isolated from Chromium Polluted Soil. J. Chem. Technol. Biotechnol. 2012, 87, 271–279. [Google Scholar] [CrossRef]
- Shanmugam, B.K.; Easwaran, S.N.; Mohanakrishnan, A.S.; Kalyanaraman, C.; Mahadevan, S. Biodegradation of Tannery Dye Effluent Using Fenton’s Reagent and Bacterial Consortium: A Biocalorimetric Investigation. J. Environ. Manag. 2019, 242, 106–113. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Senthil Velan, T.; Mandal, A.B. Biological Method for Decolourisation of an Azo Dye: Clean Technology to Reduce Pollution Load in Dye Waste Water. Clean Technol. Environ. Policy 2012, 14, 565–572. [Google Scholar] [CrossRef]
- Sundar, K.; Sadiq, I.M.; Mukherjee, A.; Chandrasekaran, N. Bioremoval of Trivalent Chromium Using Bacillus Biofilms through Continuous Flow Reactor. J. Hazard. Mater. 2011, 196, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Khalid, A.; Mahmood, T.; Arshad, M.; Ahmad, R. Potential of Newly Isolated Bacterial Strains for Simultaneous Removal of Hexavalent Chromium and Reactive Black-5 Azo Dye from Tannery Effluent. J. Chem. Technol. Biotechnol. 2013, 88, 1506–1513. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Selvaraj, H.; George, H.S.; Sundaram, M.; Khaleel, T.M. A Hybrid Treatment Process for Product Recycling from Tannery Process Effluent and Soak Liquor. J. Environ. Chem. Eng. 2020, 8, 103516. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Narenkumar, J.; Selvi, A.; Murugan, K.; Babujanarthanam, R.; Rajasekar, A. Treatment of Soak Liquor and Bioelectricity Generation in Dual Chamber Microbial Fuel Cell. Environ. Sci. Pollut. Res. 2018, 25, 11424–11430. [Google Scholar] [CrossRef]
- Muneeb, M.; Rashid, M.; Javid, A.; Bukhari, S.M.; Ali, W.; Hasan, A.; Akmal, M.; Hussain, A. Concomitant Treatment of Tannery and Paper Mill Effluents Using Extremely Metal-Tolerant Sulphate-Reducing Bacteria. Environ. Process. 2020, 7, 243–253. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Selvaraj, H.; Sundaram, M. Electrochemical Oxidation with the Aerobic Pretreatment Process for Sulfate-Rich Tannery Effluent. Environ. Sci. Pollut. Res. 2019, 26, 12194–12204. [Google Scholar] [CrossRef] [PubMed]
- Elahi, A.; Ajaz, M.; Rehman, A.; Vuilleumier, S.; Khan, Z.; Hussain, S.Z. Isolation, Characterization, and Multiple Heavy Metal-Resistant and Hexavalent Chromium-Reducing Microbacterium testaceum B-HS2 from Tannery Effluent. J. King Saud Univ. Sci. 2019, 31, 1437–1444. [Google Scholar] [CrossRef]
- Huang, G.; Ou, L.; Pan, F.; Wang, Y.; Fan, G.; Liu, G.; Wang, W. Isolation of a Novel Heterotrophic Nitrification–Aerobic Denitrification Bacterium Serratia Marcescens CL1502 from Deep-Sea Sediment. Environ. Eng. Sci. 2017, 34, 453–459. [Google Scholar] [CrossRef]
- Chaudhuri, G.; Dey, P.; Dalal, D.; Venu-Babu, P.; Thilagaraj, W.R. A Novel Approach to Precipitation of Heavy Metals from Industrial Effluents and Single-Ion Solutions Using Bacterial Alkaline Phosphatase. Water Air Soil Pollut. 2013, 224, 1625. [Google Scholar] [CrossRef]
- Pire-Sierra, M.C.; Cegarra-Badell, D.D.; Carrasquero-Ferrer, S.J.; Angulo-Cubillan, N.E.; Díaz-Montiel, A.R. Nitrogen and COD removal from tannery wastewater using biological and physicochemical treatments. Rev. Fac. Ing. Univ. Antioq. 2016, 80, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-S.; Ekpeghere, K.I.; Ha, S.-Y.; Kim, B.-S.; Song, B.; Kim, J.-T.; Kim, H.-G.; Koh, S.-C. Full-Scale Biological Treatment of Tannery Wastewater Using the Novel Microbial Consortium BM-S-1. J. Environ. Sci. Health Part A 2014, 49, 355–364. [Google Scholar] [CrossRef]
- Vijayaraj, A.S.; Mohandass, C.; Joshi, D.; Rajput, N. Effective Bioremediation and Toxicity Assessment of Tannery Wastewaters Treated with Indigenous Bacteria. 3 Biotech 2018, 8, 428. [Google Scholar] [CrossRef]
- Garg, S.K.; Garg, S.; Tripathi, M.; Singh, K. Microbial Treatment of Tannery Effluent by Augmenting Psychrotrophic Pseudomonas putida Isolate. Environ. Pollut. Prot. 2018, 3, 23–39. [Google Scholar] [CrossRef]
- Uddin, M.J.; Jeong, Y.-K.; Lee, W. Microbial Fuel Cells for Bioelectricity Generation through Reduction of Hexavalent Chromium in Wastewater: A Review. Int. J. Hydrogen Energy 2021, 46, 11458–11481. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An Overview of Microbial Fuel Cell Usage in Wastewater Treatment, Resource Recovery and Energy Production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, V.; Pisutpaisal, N. Simultaneous Pollution Treatment and Electricity Generation of Tannery Wastewater in Air-Cathode Single Chamber MFC. Int. J. Hydrogen Energy 2016, 41, 15632–15637. [Google Scholar] [CrossRef]
- Chen, F.; Zeng, S.; Luo, Z.; Ma, J.; Zhu, Q.; Zhang, S. A Novel MBBR–MFC Integrated System for High-Strength Pulp/Paper Wastewater Treatment and Bioelectricity Generation. Sep. Sci. Technol. 2020, 55, 2490–2499. [Google Scholar] [CrossRef]
- Naina Mohamed, S.; Ajit Hiraman, P.; Muthukumar, K.; Jayabalan, T. Bioelectricity Production from Kitchen Wastewater Using Microbial Fuel Cell with Photosynthetic Algal Cathode. Bioresour. Technol. 2020, 295, 122226. [Google Scholar] [CrossRef]
- Amutha, R.; Josiah, J.J.M.; Adriel Jebin, J.; Jagannathan, P.; Berchmans, S. Chromium Hexacyanoferrate as a Cathode Material in Microbial Fuel Cells. J. Appl. Electrochem. 2010, 40, 1985–1990. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Wastewater Treatment and Electricity Production in a Microbial Fuel Cell with Cu–B Alloy as the Cathode Catalyst. Catalysts 2019, 9, 572. [Google Scholar] [CrossRef] [Green Version]
- Tanikkul, P.; Pisutpaisal, N. Membrane-Less MFC Based Biosensor for Monitoring Wastewater Quality. Int. J. Hydrogen Energy 2018, 43, 483–489. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, Y.; Li, F.; Feng, C. In-Situ Cr(VI) Reduction with Electrogenerated Hydrogen Peroxide Driven by Iron-Reducing Bacteria. Bioresour. Technol. 2011, 102, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, P.; Chhabra, M. Simultaneous Chromium Removal and Power Generation Using Algal Biomass in a Dual Chambered Salt Bridge Microbial Fuel Cell. J. Bioremediat. Biodegrad. 2013, 4, 190. [Google Scholar] [CrossRef] [Green Version]
- Sophia, A.C.; Saikant, S. Reduction of Chromium(VI) with Energy Recovery Using Microbial Fuel Cell Technology. J. Water Process Eng. 2016, 11, 39–45. [Google Scholar] [CrossRef]
- Watanabe, K. Recent Developments in Microbial Fuel Cell Technologies for Sustainable Bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Adeloju, S.B.; Ghosh, P.C. Landfill Leachate: A Promising Substrate for Microbial Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 23794–23798. [Google Scholar] [CrossRef] [Green Version]
- Rittmann, B.E. Opportunities for Renewable Bioenergy Using Microorganisms. Biotechnol. Bioeng. 2008, 100, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Feng, C.; Ni, J.; Zhang, J.; Huang, W. Simultaneous Reduction of Vanadium (V) and Chromium (VI) with Enhanced Energy Recovery Based on Microbial Fuel Cell Technology. J. Power Sources 2012, 204, 34–39. [Google Scholar] [CrossRef]
- Tandukar, M.; Huber, S.J.; Onodera, T.; Pavlostathis, S.G. Biological Chromium(VI) Reduction in the Cathode of a Microbial Fuel Cell. Environ. Sci. Technol. 2009, 43, 8159–8165. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.-H.; Show, P.L. Potential Utilization of Bioproducts from Microalgae for the Quality Enhancement of Natural Products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef]
- Ballén-Segura, M.; Hernández Rodríguez, L.; Parra Ospina, D.; Vega Bolaños, A.; Pérez, K. Using Scenedesmus sp. for the Phycoremediation of Tannery Wastewater. Tecciencia 2016, 11, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Sydney, E.B.; Schafranski, K.; Barretti, B.R.V.; Sydney, A.C.N.; Zimmerman, J.F.D.; Cerri, M.L.; Mottin Demiate, I. Biomolecules from Extremophile Microalgae: From Genetics to Bioprocessing of a New Candidate for Large-Scale Production. Process Biochem. 2019, 87, 37–44. [Google Scholar] [CrossRef]
- Sadvakasova, A.K.; Akmukhanova, N.R.; Bolatkhan, K.; Zayadan, B.K.; Usserbayeva, A.A.; Bauenova, M.O.; Akhmetkaliyeva, A.E.; Allakhverdiev, S.I. Search for New Strains of Microalgae-Producers of Lipids from Natural Sources for Biodiesel Production. Int. J. Hydrogen Energy 2019, 44, 5844–5853. [Google Scholar] [CrossRef]
- Das, C.; Ramaiah, N.; Pereira, E.; Naseera, K. Efficient Bioremediation of Tannery Wastewater by Monostrains and Consortium of Marine Chlorella sp. and Phormidium sp. Int. J. Phytoremed. 2018, 20, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and Phosphate Removal from Wastewater with a Mixed Microalgae and Bacteria Culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Kuo, E.-W.; Nagarajan, D.; Ho, S.-H.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Cultivating Chlorella sorokiniana AK-1 with Swine Wastewater for Simultaneous Wastewater Treatment and Algal Biomass Production. Bioresour. Technol. 2020, 302, 122814. [Google Scholar] [CrossRef]
- Msanne, J.; Polle, J.; Starkenburg, S. An Assessment of Heterotrophy and Mixotrophy in Scenedesmus and Its Utilization in Wastewater Treatment. Algal Res. 2020, 48, 101911. [Google Scholar] [CrossRef]
- Vidyalaxmi; Kaushik, G.; Raza, K. Potential of Novel Dunaliella salina from Sambhar Salt Lake, India, for Bioremediation of Hexavalent Chromium from Aqueous Effluents: An Optimized Green Approach. Ecotoxicol. Environ. Saf. 2019, 180, 430–438. [Google Scholar] [CrossRef]
- Srinivasan, S.V.; Rema, T.; Chitra, K.; Sri Balakameswari, K.; Suthanthararajan, R.; Uma Maheswari, B.; Ravindranath, E.; Rajamani, S. Decolourisation of Leather Dye by Ozonation. Desalination 2009, 235, 88–92. [Google Scholar] [CrossRef]
- Ansari, F.A.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Evaluation of Various Cell Drying and Disruption Techniques for Sustainable Metabolite Extractions from Microalgae Grown in Wastewater: A Multivariate Approach. J. Clean. Prod. 2018, 182, 634–643. [Google Scholar] [CrossRef]
- Behera, M.; Dhali, D.; Chityala, S.; Mandal, T.; Bhattacharya, P.; Mandal, D.D. Evaluation of Performance of Planococcus sp. TRC1 an Indigenous Bacterial Isolate Monoculture as Bioremediator for Tannery Effluent. Desalin. Water Treat. 2016, 57, 13213–13224. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Selvaraju, M. Heavy Metal Induced Antioxidant Defense System of Green Microalgae and Its Effective Role in Phycoremediation of Tannery Effluent. Pak. J. Biol. Sci. 2012, 15, 1056–1062. [Google Scholar] [CrossRef]
- Rose, P.; Dunn, K. A High Rate Ponding Unit Operation Linking Treatment of Tannery Effluent and Arthrospira (Spirulina) Biomass Production. 1: Process Development. Biomass Bioenergy 2013, 51, 183–188. [Google Scholar] [CrossRef]
- Ardila, L.; Godoy, R.; Montenegro, L. Sorption Capacity Measurement of Chlorella vulgaris and Scenedesmus acutus to Remove Chromium from Tannery Waste Water. Proc. IOP Conf. Ser. Earth Environ. Sci. 2017, 83, 12031. [Google Scholar] [CrossRef] [Green Version]
- Nagabalaji, V.; Sivasankari, G.; Srinivasan, S.V.; Suthanthararajan, R.; Ravindranath, E. Nutrient removal from synthetic and secondary treated sewage and tannery wastewater through phycoremediation. Environ. Technol. 2019, 40, 784–792. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Harilal, C.C.; Selvaraju, M. Phycoremediation Resultant Lipid Production and Antioxidant Changes in Green Microalgae Chlorella sp. Int. J. Phytoremed. 2018, 20, 1144–1151. [Google Scholar] [CrossRef]
- Saranya, D.; Shanthakumar, S. Effect of culture conditions on biomass yield of acclimatized microalgae in ozone pre-treated tannery effluent: A simultaneous exploration of bioremediation and lipid accumulation potential. J. Environ. Manag. 2020, 273, 111129. [Google Scholar] [CrossRef] [PubMed]
- da Fontoura, J.T.; Rolim, G.S.; Mella, B.; Farenzena, M.; Gutterres, M. Defatted Microalgal Biomass as Biosorbent for the Removal of Acid Blue 161 Dye from Tannery Effluent. J. Environ. Chem. Eng. 2017, 5, 5076–5084. [Google Scholar] [CrossRef]
- Sforza, E.; Kumkum, P.; Barbera, E.; Kumar, S. Bioremediation of industrial effluents: How a biochar pretreatment may increase the microalgal growth in tannery wastewater. J. Water Process Eng. 2020, 37, 101431. [Google Scholar] [CrossRef]
- Salama, E.S.; Kurade, M.B.; Abou-Shanab, R.A.I.; El-Dalatony, M.M.; Yang, I.S.; Min, B.; Jeon, B.H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Gendy, T.S.; El-Temtamy, S.A. Commercialization potential aspects of microalgae for biofuel production: An overview. Egypt. J. Pet. 2013, 22, 43–51. [Google Scholar] [CrossRef] [Green Version]




| pH | COD (mg*L−1) | BOD (mg*L−1) | TDS (mg*L−1) | Cr (mg*L−1) | NH3–N (mg*L−1) | PO4 (mg*L−1) | Reference |
|---|---|---|---|---|---|---|---|
| n/a | 17,683 ± 1500 | 6000 ± 300 | 10,000 ± 800 | n/a | 4500 | 4100 | [9] |
| 7.5 | 4000 | 1400 | n/a | n/a | 343 | 6.6 | [13] |
| 3.4–3.7 | 5250–9600 | n/a | 38,200–39,400 | 2705–3800 | 115–136 | n/a | [35] |
| 3.5–4 | 6800 | n/a | n/a | n/a | n/a | 1.76 | [36] |
| 8.49 ± 0.2 | 322 ± 28.6 | 160 ± 15.8 | 3491.3 ± 239.4 | 1445 ± 67.9 | n/a | 5.7 ± 0.2 | [37] |
| 7.9 | 4155 | – | n/a | n/a | 485 | 524 | [38] |
| 8.9 ± 0.1 | 4500 ± 329 | 400 ± 36 | 5900 ± 391 | n/a | 129.69 ± 7.75 | 194.61 ± 9.8 | [39] |
| 5.84 ± 0.02 | 198.60 ± 0.23 | 6.5 ± 0.10 | 72,400 ± 0.10 | 0.83 ± 0.28 | n/a | 294.4 ± 0.22 | [40] |
| 8.45 ± 0.18 | 1300.00 ± 10.0 | 680.00 ± 20.0 | 3850.00 ± 10.0 | 7.39 ± 0.03 | 12.3 ± 0.3 | 12.5 ± 0.5 | [41] |
| 7.45 ± 0.00 | 4000.00 ± 51.20 | n/a | 4333.33 ± 288.70 | 3.22 | 2734.16 ± 1.12 | 6.01 ± 0.05 | [42] |
| 4.13 | 5485 | 90 | n/a | 2007.08 | n/a | n/a | [44] |
| 4–9 | 1235 | 450 | n/a | 128.8 | n/a | n/a | [45] |
| 11.64 ± 0.53 | 7200 ± 1090 | 1250 ± 380 | n/a | 7.02 ± 0.76 | n/a | n/a | [46] |
| 6.25 | 11,800 | 1200 | n/a | 32.2 | n/a | n/a | [48] |
| 8.36 | 5308.4 | 1952.5 | 1578 | 123.1897 | n/a | n/a | [49] |
| 7.5 | 4291 mg/L | 2102.60 | n/a | n/a | n/a | n/a | [50] |
| 3.78 | 1980 | n/a | n/a | 3060 | n/a | n/a | [56] |
| 8.67 ± 3.5 | 7273 ± 536 | 3120.6 ± 172 | n/a | 28.47 ± 5 | 112.2 ± 24 | n/a | [51] |
| 11.3 ± 0.1 | 3000 ± 100 | n/a | n/a | 50 ± 3 | n/a | n/a | [52] |
| 3.9 ± 0.1 | 4321 ± 21.2 | 3200 ± 77 | 42,200 ± 100 | 2920.2 ± 0.7 | n/a | n/a | [53] |
| 8.0 ± 0.4 | 5634 ± 245 | 2910 ± 341 | 10,560 ± 978 | 134 ± 5.8 | n/a | n/a | [54] |
| 8.6 ± 0.1 | 12,560 ± 1880 | 4860 ± 129 | 18,250 ± 1825 | n/a | n/a | n/a | [55] |
| 3.17 | 1130 | n/a | n/a | 1640 | n/a | n/a | [57] |
| 6.5 | 2530 | n/a | 822 | 57 | 57 | n/a | [58] |
| 8.8 | 2780 | 1225 | n/a | 8.2 | n/a | n/a | [59] |
| 9.3−12.1 | 1500 ± 400 | n/a | n/a | 360 ± 110 | n/a | n/a | [60] |
| 8.7 ± 0.2 | 2412 ± 145 | 649.3 ± 39.3 | 2355 ± 85 | 8.11 ± 4.86 | n/a | n/a | [61] |
| 9 | 17,600 | n/a | 6900 | 120 | n/a | 916 | [62] |
| 4.0 ± 0.12 | 300 ± 2.08 | 250 ± 1.62 | 19.426 ± 3.06 | 25 | n/a | n/a | [63] |
| 6.85 | 987 | 580 | 1185.4 | 12.4 | n/a | n/a | [64] |
| 4.12 | 3280 | n/a | n/a | 147.4 | n/a | n/a | [74] |
| Process | Operating Conditions | Evaluated Parameters | Efficiency | Reference |
|---|---|---|---|---|
| Cavitation | The amount of energy dissipated in 250 mL was 0.122 W*mL−1 | COD | 87% | [50] |
| Fenton | V: 50 mL, T: 25 ± 0.1 °C, agitation: 150 rpm, FeSO4: 1–5 g L−1, time: 5–300 min; H2O2/COD ratio (w/w): 0.5–1.0. | COD | 58.4% | [95] |
| V: 500 mL, pH: 3, T: 40–45 °C, H2O2: 0.15–0.6 g L−1, FeSO4: 500–750 mg L−1, time: 0–30 min. | COD | 68% | [96] | |
| 3V: 300 mL, agitation: 150 rpm, time: 60 min; Fe2+ dosage: 0–20 mg L−1, pH: 3–7, H2O2 dosage: 50–100 mg L−1. | COD Color Turbidity Sludge | COD: 80% Color: 90% Turbidity: 95% Sludge: 70% | [98] | |
| Fenton + NaOCl and Fenton + (NH4)2S2O8 | V: 100 mL, pH: 3.5, agitation: 200 rpm, Fe2+ dosage: 11.5 mg/g DS, H2O2 dosage: 167.0 mg/g DS, time: 12 min. | Cr | 73.3% | [97] |
| Photo-Fenton | V: 500 mL, solar irradiation: 5 h; Fe2+: 0.4–0.5 g L−1; H2O2: 15–30 g L−1, pH: 3, time: 2 h. | COD TDS | COD: 90% TSS: 50% | [100] |
| Ozone | V: 2500 L, flow rate: 2 m3 h−1; O3 dosage: 150 g m−3, time: 60 min, pH: 6.8. | COD TSS TKN Color | COD: 97% TSS: 96% TKN: 91% Color: 96% | [101] |
| V: 5 L, pH: 4–7–9, O3 dosage: 1.6 mg L−1, time: 10–20–30–40–50 min. | Color | 97% in a time of 20 min and a pH of 7 | [102] | |
| V: 3 L, pH: 3–6–9, Ozone flow rate: 1 and 8 g h −1. time: 10–20–40–60–90−120 min, T: 27 °C | COD | COD: 70% | [104] | |
| Fenton and Ozone | V: 0.5 L, Fe2+ concentration: 120 to 300 mg L−1, concentration of H2O2: 600–2000 mg L−1, pH: 4, Ozone flow: 1 L min−1. | COD | COD: 60–70% | [105] |
| Ozone coupled with phycoremediation | V: 1 L, pH: 3.7–6–9, ozone flow rate: 2–4–6 g h−1, time: 10–20–40–60–90−120 min. | COD, сolor, Cr, NH4, PO4, TDS | COD: 84% Color: 60% Cr: 97% NH4–N: 82% PO4–P: 100% TDS: 10% | [43] |
| Electrochemical | V: 2 L, total surface area: 427.84 cm2; pH: 3–9; salt concentration: 10–40 g L−1 NaCl; time: 120 min. | COD | COD: 89% to 0.012 A cm−2 pH: 9, salt concentration: 30 g*L−1 NaCl | [107] |
| V: 1.15 L, total surface area: 69.75 cm2, pH: 2−11; current density: 3.5–70 A cm−2; time: 10–70 min. | OD | COD: 62% in a range of pH 3–5 and time: 10 min | [83] | |
| Electrochemical/photo-Fenton/Fenton | V: 1.5 L, pH 8.3; current density: 68 mA cm−2; currents and voltages: 0–10 A and 0–30 V; t: 5–60 min. | COD, color, turbidity | COD: 99% Turbidity: 98% TSS: 65% | [103] |
| V: 4 L, pH: 3; anode and cathode electrode area: 64 cm2; time: 180 min. | COD, color | COD: 90% Color: 86% | [108] | |
| V: 500 mL, pH: 3.0, H2O2 concentration: 0.5 Mm; Fe2+ concentration: 0.50 mM. | COD, color | Color: 97% COD: 95% | [109] | |
| Electrocoagulation combined with photoreactor UVC | V: 0.2 L, UV lamp wavelength: 254 nm and 185 nm; electric current: 100–600 mA; time: 10–30 min. | COD, Cr | COD: 99.52% Cr: 98.27% | [110] |
| Photocatalysis | Air flow: 140 N cm3 min−1; four UV lamps: power: 8 W, wavelength: 350 nm; photon flux: 25 mW/cm2. | COD | The ZnO_ac1 photocatalyst achieved a COD removal of 70% in 180 min of irradiation | [111] |
| V: 5 L; without pH adjustment; time: 5 h in PTR; exposed directly to sunlight. | COD, Cr | COD: 82.26% Cr: 76.48% | [112] |
| AOP Type | EEO (kWh m−3 Order−1) | EEM (kWh g−1) | Cost (US$ m−3) | References |
|---|---|---|---|---|
| O3 | 0.3 | 495 | 11.3 | [114,122,124,125,126,127,128,129,130] |
| O3/H2O2 | 0.2 | - | 8.6 | |
| UV/O3 | 225.25 | 111.56 | 6 | |
| Photo-Fenton | 12 | - | 64.13 | |
| Photoelectro-Fenton | 132.6 | 0.125 | 8.4–66.22 | |
| Electro-Fenton (EF) | 127.2 | 0.235 | 8.48 | |
| UV/US/H2O2 | 39.76 | 167 | 4.49 | |
| Ultrasound | 800–8000 | 11,993 | 55.14 | |
| Photocatalysis | 3654.68 | 21,129.15 | 2.8 | |
| Electrocoagulation | 59.4 | 0.060 | 3.94 |
| Microorganism | Removal Efficiency | MFC Performance | Reference |
|---|---|---|---|
| Bacillus sp. | COD: 88% | 120 mA/m2 and 7 mW/m2 | [155] |
| Anaerobic microbial consortium | COD: 48.5% | 44.2 and 52.1 mW/m2 | [156] |
| Chlorococcum sp. Synechococcus sp. | COD: 73.5% (Chlorococcum sp.) 69.4% (Synechococcus sp.) | Chlorococcum sp.: 32.1 ± 0.5 and 27.2 ± 0.5 mW/m2 Synechococcus sp.: 42.5 ± 0.5 and 37.2 ± 0.3 mW/m2 | [157] |
| Activated sludge consortium | NO3−: 87% COD: 90% | 0.35 mA·cm−2 and power level of 6.11 mW | [159] |
| Anaerobic sludge | COD: 98% | 88 mW/m2 and 408 mA*m−2 | [160] |
| Shewanella decolorationis S12, Klebsiella pneumoniae L17 | COD: 42.5% | 52.1 mW/cm2 with an air bubbling cathode 6.8 mW/cm2 with a nitrogen bubbling cathode | [161] |
| Algae biomass | COD: 72–95% Cr: 98% | 221 mV to 760 mV | [162] |
| Anaerobic microbial consortium | Cr6+: 95% | 89 ± 3 mW/m2 | [163] |
| Adapted microbial consortium | Cr: 71.4% | 970.2 ± 20.6 mW/m2 | [167] |
| Trichococcus pasteurii Pseudomonas aeruginosa | COD: 98% | 55.5 mW/m2 | [168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbina-Suarez, N.A.; Machuca-Martínez, F.; Barajas-Solano, A.F. Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater. Molecules 2021, 26, 3222. https://doi.org/10.3390/molecules26113222
Urbina-Suarez NA, Machuca-Martínez F, Barajas-Solano AF. Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater. Molecules. 2021; 26(11):3222. https://doi.org/10.3390/molecules26113222
Chicago/Turabian StyleUrbina-Suarez, Néstor Andrés, Fiderman Machuca-Martínez, and Andrés F. Barajas-Solano. 2021. "Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater" Molecules 26, no. 11: 3222. https://doi.org/10.3390/molecules26113222
APA StyleUrbina-Suarez, N. A., Machuca-Martínez, F., & Barajas-Solano, A. F. (2021). Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater. Molecules, 26(11), 3222. https://doi.org/10.3390/molecules26113222