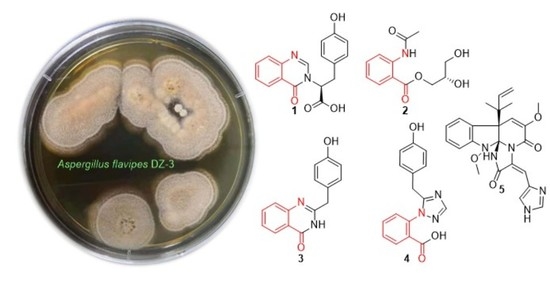
Asperflaloids A and B from Aspergillus flavipes DZ-3, an Endophytic Fungus of Eucommia ulmoides Oliver
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Fungal Strain and Identification
3.3. Fermentation, Extraction, and Isolation
3.4. α-Glucosidase Assay
3.5. Antioxidant Activity
3.6. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Spiteller, P. Chemical ecology of fungi. Nat. Prod. Rep. 2015, 32, 971–993. [Google Scholar] [CrossRef]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- El-hawary, S.S.; Moawad, A.S.; Bahr, H.S.; Abdelmohsen, U.R.; Mohammed, R. Natural product diversity from the endophytic fungi of the genus Aspergillus. RSC Adv. 2020, 10, 22058–22079. [Google Scholar] [CrossRef]
- Sanchez, J.F.; Somoza, A.D.; Keller, N.P.; Wang, C.C.C. Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 2012, 29, 351–371. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.C.; Chen, C.M.; Tong, Q.Y.; Yang, J.; Wei, G.Z.; Xue, Y.B.; Wang, J.P.; Luo, Z.W.; Zhang, Y.H. Asperflavipine A: A cytochalasan heterotetramer uniquely defined by a highly complex tetradecacyclic ring system from Aspergillus flavipes QCS12. Angew. Chem. Int. Edit. 2017, 56, 5242–5246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.C.; Chen, C.M.; Tong, Q.Y.; Li, X.N.; Yang, J.; Xue, Y.B.; Luo, Z.W.; Wang, J.P.; Yao, G.M.; Zhang, Y.H. Epicochalasines A and B: Two bioactive merocytochalasans bearing caged epicoccine dimer units from Aspergillus flavipes. Angew. Chem. Int. Edit. 2016, 55, 3486–3490. [Google Scholar] [CrossRef]
- Zhang, X.T.; Yang, L.; Wang, W.J.; Wu, Z.D.; Wang, J.P.; Sun, W.G.; Li, X.N.; Chen, C.M.; Zhu, H.C.; Zhang, Y.H. Flavipesines A and B and asperchalasines E-H: Cytochalasans and merocytochalasans from Aspergillus flavipes. J. Nat. Prod. 2019, 82, 2994–3001. [Google Scholar] [CrossRef]
- Wang, C.; Wu, X.H.; Bai, H.L.; Zaman, K.; Hou, S.B.; Saito, J.; Wongwiwatthananukit, S.; Kim, K.S.; Cao, S.G. Antibacterial and NF-kappa B inhibitory lumazine peptides, aspochalasin, gamma-butyrolactone derivatives, and cyclic peptides from a hawaiian Aspergillus flavipes. J. Nat. Prod. 2020, 83, 2233–2240. [Google Scholar] [CrossRef]
- Jiao, W.H.; Xu, Q.H.; Ge, G.B.; Shang, R.Y.; Zhu, H.R.; Liu, H.Y.; Cui, J.; Sun, F.; Lin, H.W. Flavipesides A–C, PKS-NRPS hybrids as pancreatic lipase inhibitors from a marine sponge symbiotic fungus Aspergillus flavipes 164013. Org. Lett. 2020, 22, 1825–1829. [Google Scholar] [CrossRef]
- Machado, F.P.; Kumla, D.; Pereira, J.A.; Sousa, E.; Dethoup, T.; Freitas-Silva, J.; Costa, P.M.; Mistry, S.; Silva, A.M.S.; Kijjoa, A. Prenylated phenylbutyrolactones from cultures of a marine sponge-associated fungus Aspergillus flavipes KUFA1152. Phytochemistry 2021, 185, 112709. [Google Scholar] [CrossRef]
- Li, Q.; Xu, W.; Fan, R.; Zhang, J.; Li, Y.; Wang, X.; Han, S.; Liu, W.; Pan, M.; Cheng, Z. Penithoketone and penithochromones A-L, polyketides from the deep-sea-derived fungus Penicillium thomii YPGA3. J. Nat. Prod. 2020, 83, 2679–2685. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y.; Xu, W.; Liu, W.; Liu, L.; Zhu, D.; Kang, Y.; Luo, Z.; Li, Q. Three new cyclopiane-type diterpenes from a deep-sea derived fungus Penicillium sp. YPGA11 and their effects against human esophageal carcinoma cells. Bioorg. Chem. 2019, 91, 103129. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y.; Liu, W.; Liu, L.; Liu, J.; Yuan, W.; Luo, Z.; Xu, W.; Li, Q. Butenolide derivatives with α-glucosidase inhibitions from the deep-sea-derived fungus Aspergillus terreus YPGA10. Mar. Drugs 2019, 17, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, W.; Han, S.; Zhang, J.; Xu, W.; Li, Q.; Cheng, Z. Penitholabene, a rare 19-nor labdane-type diterpenoid from the deep-sea-derived fungus Penicillium thomii YPGA3. Fitoterapia 2020, 146, 104691. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Liu, W.; Xu, W.; Zeng, X.; Cheng, Z.B.; Li, Q. Aspterrics A and B, new sesquiterpenes from deep sea-derived fungus Aspergillus terreus YPGA10. Rec. Nat. Prod. 2020, 14, 18–22. [Google Scholar] [CrossRef]
- Casati, S.; Ciuffreda, P.; Santaniello, E. Synthesis of enantiomerically pure (R)-and (S)-1-benzoyloxypropane-2,3-diol and revision of the stereochemical outcome of the Candida antarctica lipase-catalyzed benzoylation of glycerol. Tetrahedron Asymmetry 2011, 22, 658–661. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Niu, S.; Zhang, H.; Liu, X.Z.; Che, Y.S. N-hydroxypyridones, phenylhydrazones, and a quinazolinone from Isaria farinosa. J. Nat. Prod. 2011, 74, 32–37. [Google Scholar] [CrossRef]
- Li, C.S.; An, C.Y.; Li, X.M.; Gao, S.S.; Cui, C.M.; Sun, H.F.; Wang, B.G. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus Penicillium paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.F.; Kim, D.S.; Choi, H.O.; Son, B.W. Indolyl alkaloid derivatives, Nb-acetyltryptamine and oxaline from a marine-derived fungus. Arch. Pharm. Res. 2003, 26, 21–23. [Google Scholar] [CrossRef]
- Biren, K.J.; James, B.G.; Donald, T.W. Bioactive natural products from a sclerotium-colonizing isolate of Humicola fuscoatra. J. Nat. Prod. 2002, 65, 1734–1737. [Google Scholar]
- Li, L.Y.; Yi, J.L.; Cai, J.; Zhou, X.M.; Chen, L.; Zhuo, X.; Lai, X.Y. Two new bioactive secondary metabolites from the endophytic fungus Talaromyces assiutensis JTY2. Nat. Prod. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.E.; Storz, T.; Colyer, J.T.; Thiel, O.R.; Seran, M.D.; Larsen, R.D.; Murry, J.A. Iodide-catalyzed reductions: Development of a synthesis of phenylacetic acids. J. Org. Chem. 2011, 76, 9519–9524. [Google Scholar] [CrossRef]
- Agerbirk, N.; Warwick, S.I.; Hansen, P.R.; Olsen, C.E. Sinapis phylogeny and evolution of glucosinolates and specific nitrile degrading enzymes. Phytochemistry 2008, 69, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.S.; Narsaiah, A.V. An efficient asymmetric synthesis of (S)-atenolol: Using hydrolytic kinetic resolution. Bioorg. Med. Chem. 2005, 13, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, W.; Fan, R.; Han, S.; Li, Y.; Cui, X.; Zhang, J.; Wu, Y.; Lv, X.; Zhang, Y.; et al. Terpenoids from the deep-sea-derived fungus Penicillium thomii YPGA3 and their bioactivities. Mar. Drugs 2020, 18, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, P.; Liu, Z.; Chen, Y.; Cai, R.; Chen, G.; She, Z. Secondary metabolites with α-glucosidase inhibitory activity from the mangrove fungus Mycosphaerella sp. SYSU-DZG01. Mar. Drugs 2019, 17, 483. [Google Scholar] [CrossRef] [Green Version]
- Sybyl Software, Version X 2.0; Tripos Associates Inc.: St. Louis, MO, USA, 2013.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev. C 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef] [PubMed]

 ) and HMBC (
) and HMBC (  ) correlations of 1 and 2.
) correlations of 1 and 2.


| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 2 | 7.94, s | 148.6 | 171.6 | |
| 3 | 2.21, s | 24.9 | ||
| 4 | 162.3 | 169.1 | ||
| 4a | 122.7 | 118.1 | ||
| 5 | 8.20, d (7.9) | 127.5 | 8.11, dd (8.0, 1.6) | 132.2 |
| 6 | 7.54, dd (7.9, 7.5) | 128.6 | 7.18, dd (8.0, 7.9, 1.0) | 124.3 |
| 7 | 7.80, dd (8.1, 7.5) | 135.9 | 7.57, dd (8.3, 7.9, 1.6) | 135.2 |
| 8 | 7.62, d (8.1) | 127.8 | 8.45, dd (8.3, 1.0) | 122.1 |
| 8a | 148.5 | 141.8 | ||
| 1′ | 3.44, dd (14.4, 4.8); 3.53, dd (14.4, 10.9) | 35.6 | 4.45, dd (11.4, 4.1); 4.34, dd (11.4, 6.3) | 67.5 |
| 2′ | 128.4 | 3.99, m | 71.0 | |
| 3′ | 6.93, d (8.3) | 131.1 | 3.65, d (5.7) | 64.0 |
| 4′ | 6.60, d (8.3) | 116.5 | ||
| 5′ | 157.5 | |||
| 6′ | 6.60, d (8.3) | 116.5 | ||
| 7′ | 6.93, d (8.3) | 131.1 | ||
| 8′ | 5.40, dd (10.9, 4.8) | 62.3 | ||
| 9′ | 172.2 | |||
| Compounds | α-Glucosidase Inhibitory | Antioxidant | |
|---|---|---|---|
| IC50 (μM) | % Inhibition (500 μM) | IC50 (μM) | |
| 1 | − | <50 | − |
| 2 | − | <50 | − |
| 3 | 750.8 | <50 | − |
| 4 | − | <50 | − |
| 5 | − | <50 | − |
| 6 | − | <50 | − |
| 7 | − | 96.6 | 14.4 |
| 8 | − | 92 | 27.1 |
| 9 | − | <50 | − |
| 10 | − | 64.9 | 339.3 |
| 11 | − | <50 | − |
| 12 | − | <50 | − |
| Acarbose a | 1330 | ||
| Vitamin C a | 96.4 | 26.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Liu, Y.; Yang, F.; Han, S.; Zhang, J.; Yang, H.; Cheng, Z.; Li, Q. Asperflaloids A and B from Aspergillus flavipes DZ-3, an Endophytic Fungus of Eucommia ulmoides Oliver. Molecules 2021, 26, 3514. https://doi.org/10.3390/molecules26123514
Liu W, Liu Y, Yang F, Han S, Zhang J, Yang H, Cheng Z, Li Q. Asperflaloids A and B from Aspergillus flavipes DZ-3, an Endophytic Fungus of Eucommia ulmoides Oliver. Molecules. 2021; 26(12):3514. https://doi.org/10.3390/molecules26123514
Chicago/Turabian StyleLiu, Wan, Yu Liu, Fan Yang, Shouye Han, Jia Zhang, Hui Yang, Zhongbin Cheng, and Qin Li. 2021. "Asperflaloids A and B from Aspergillus flavipes DZ-3, an Endophytic Fungus of Eucommia ulmoides Oliver" Molecules 26, no. 12: 3514. https://doi.org/10.3390/molecules26123514
APA StyleLiu, W., Liu, Y., Yang, F., Han, S., Zhang, J., Yang, H., Cheng, Z., & Li, Q. (2021). Asperflaloids A and B from Aspergillus flavipes DZ-3, an Endophytic Fungus of Eucommia ulmoides Oliver. Molecules, 26(12), 3514. https://doi.org/10.3390/molecules26123514