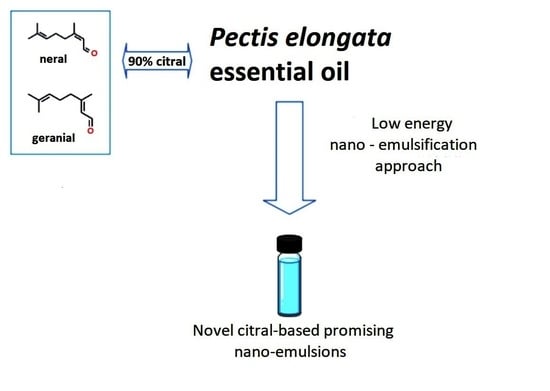
A Low Energy Approach for the Preparation of Nano-Emulsions with a High Citral-Content Essential Oil
Abstract
:1. Introduction
2. Results
2.1. Essential Oil
2.2. Nano-Emulsification and Citral Ratio
2.3. Physical Characterization and Nanodroplet Behavior
3. Discussion
4. Materials and Methods
4.1. Essential Oil
4.1.1. Obtainment by Hydrodistillation
4.1.2. Characterization by GasChromatography (GC)
4.2. Nano-Emulsion
4.2.1. Nano-Emulsification Process
Gas Chromatography Profile
Citral Ratio
4.2.2. Physical Characterization
Droplet Growth (DG)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Marques, A.M.; Kaplan, M.A.C. Preparative isolation and characterization of monoterpene isomers present in the citral-rich essential oil of Pectis brevipedunculata. J. Essent. Oil Res. 2013, 25, 210–215. [Google Scholar] [CrossRef]
- Marques, A.M.; Lima, C.H.P.; Alviano, D.S.; Alviano, C.S.; Esteves, R.L.; Kaplan, M.A.C. Traditional use, chemical composition and antimicrobial activity of Pectis brevipedunculata essential oil: A correlated lemongrass species in Brazil. Emir. J. Food Agric. 2013, 25, 798–808. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.H.L.; Andrade, E.H.A.; Maia, J.G.S. The essential oil of Pectis elongata Kunth occuring in North Brazil. Flavour Fragr. J. 2005, 20, 462–464. [Google Scholar] [CrossRef]
- De Oliveira, M.T.R.; Berbert, P.A.; Matos, C.R.R.; Mathias, L.; Moreira, R.O. Efeito da temperatura do ar de secagem sobre o teor e a composição química do óleo essencial de Pectis brevipedunculata. Quim. Nova 2011, 34, 1200–1204. [Google Scholar] [CrossRef]
- Bautista, H.P.; Pectis, L. (Compositae–Tageteae), Espécies Ocorrentes No Brasil; v. 28; Arquivos do Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 1987; pp. 20–107. [Google Scholar]
- Prudent, D.; Perineau, F.; Bessiere, J.M.; Michel, G. Analysis of the Essential Oil of Pectis elongata Kunth. From Martinique. Evaluation of its Bacteriostatic and Fungistatic Properties. J. Essent. Oil Res. 1995, 7, 63–68. [Google Scholar] [CrossRef]
- Nedeltcheva-Antonova, D.; Stoicheva, P.; Antonov, L. Chemical profiling of Bulgarian rose absolute (Rosa damascena Mill.) using gas chromatography–mass spectrometry and trimethylsilyl derivatives. Ind. Crops Prod. 2017, 108, 36–43. [Google Scholar] [CrossRef]
- Lubinska-Szczygeł, M.; Różańska, A.; Dymerski, T.; Namieśnik, J.; Katrich, E.; Gorinstein, S. A novel analytical approach in the assessment of unprocessed Kaffir lime peel and pulp as potential raw materials for cosmetic applications. Ind. Crops Prod. 2018, 120, 313–321. [Google Scholar] [CrossRef]
- Nishijima, C.M.; Ganev, E.G.; Mazzardo-Martins, L.; Martins, D.F.; Rocha, L.R.M.; Santos, A.R.S.; Hiruma-Lima, C.A. Citral: A monoterpene with prophylactic and therapeutic anti-nociceptive effects in experimental models of acute and chronic pain. Eur. J. Pharmacol. 2014, 736, 16–25. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control. 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Maghamian, N.; Goli, M.; Najarian, A. Ultrasound-assisted preparation of double nano-emulsions loaded with glycyrrhizic acid in the internal aqueous phase and skim milk as the external aqueous phase. LWT 2021, 141, 110850. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Hanedi, E.; Faten, M.; Ramzi, K.; Younes, M. The use of mucilage extracted from Opuntia ficus indica as a microencapsulating shell. J. Serb. Chem. Soc. 2021, 86, 25–38. [Google Scholar]
- Ortiz-Zamora, L.; Bezerra, D.C.; de Oliveira, H.N.S.; Duarte, J.L.; Guisado-Bourzac, F.; Chil-Núñez, I.; da Conceição, E.C.; Barroso, A.; Mourão, R.H.V.; de Faria Mota Oliveira, A.E.M.; et al. Preparation of non-toxic nano-emulsions based on a classical and promising Brazilian plant species through a low-energy concept. Ind. Crops Prod. 2020, 158. [Google Scholar] [CrossRef]
- Lima, L.A.; Ferreira-Sá, P.S.; Garcia, M.D.N.; Pereira, V.L.P.; Carvalho, J.C.T.; Rocha, L.; Fernandes, C.P.; Souto, R.N.P.; Araújo, R.S.; Botas, G.; et al. Nano-emulsions of the essential oil of Baccharis reticularia and its constituents as eco-friendly repellents against Tribolium castaneum. Ind. Crops Prod. 2021, 162. [Google Scholar] [CrossRef]
- Fernandes, C.P.; Mascarenhas, M.P.; Zibetti, F.M.; Lima, B.G.; Oliveira, R.P.R.F.; Rocha, L.; Falcão, D.Q. HLB value, an important parameter for the development of essential oil phytopharmaceuticals. Braz. J. Pharmacogn. 2013, 23, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Duarte, J.L.; Amado, J.R.R.; Oliveira, A.E.M.F.M.; Cruz, R.A.S.; Ferreira, A.M.; Souto, R.N.P.; Falcão, D.Q.; Carvalho, J.C.T.; Fernandesa, C.P. Evaluation of larvicidal activity of a nanoemulsion of Rosmarinus officinalis essential oil. Braz. J. Pharmacogn. 2015, 25, 189–192. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.E.M.F.M.; Duarte, J.L.; Cruz, R.A.S.; Conceição, E.C.; Carvalho, J.C.T.; Fernandes, C.P. Utilization of dynamic light scattering to evaluate Pterodon emarginatus oleoresin-based nanoemulsion formation by non-heating and solvent-free method. Braz. J. Pharmacogn. 2017, 27, 401–406. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Impact of lemon oil composition on formation and stability of model food and beverage emulsions. Food Chem. 2012, 134, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.F. Dimethylsulfoxide (CAS No. 67-68-5). Rev. Virtual Quim. 2011, 3, 344–352. [Google Scholar] [CrossRef]
- González, C.; Resa, J.M.; Concha, R.G.; Goenaga, J.M. Enthalpies of mixing and heat capacities of mixtures containing alcohols and n-alkanes with corn oil at 298.15 K. JAOCS J. Am. Oil Chem. Soc. 2004, 81, 817–822. [Google Scholar] [CrossRef]
- Lu, W.C.; Huang, D.W.; Wang, C.C.R.; Yeh, C.H.; Tsai, J.C.; Huang, Y.T.; Li, P.H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J. Food Drug Anal. 2018, 26, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Vallejo, R.; Guirao, P.; Rodríguez-Rojo, S.; Cocero, M.J. Use of nanoemulsions of plant essential oils as aphid repellents. Ind. Crops Prod. 2017, 110, 45–57. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Sabag, A.; Tepper-Bamnolker, P.; Chalupovich, D.; Levi-Kalisman, Y.; Eshel, D.; Porat, R.; Poverenov, E. Effective suppression of potato tuber sprouting using polysaccharide-based emulsified films for prolonged release of citral. Food Hydrocoll. 2020, 103, 105644. [Google Scholar] [CrossRef]
- Duarte, J.L.; Bezerra, D.C.; da Conceição, E.C.; Mourão, R.H.V.; Fernandes, C.P. Self-nano-emulsification of chamomile essential oil: A novel approach for a high value phytochemical. Colloids Interface Sci. Commun. 2020, 34, 100225. [Google Scholar] [CrossRef]



| RIlit | Compound | EO | NE1 | NE2 | NE3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Area (%) | RIexp | Area (%) | RIexp | Area (%) | RIexp | Area (%) | RIexp | ||
| 936 | Heptanone<5-Methyl-3-> | 0.12 | 941 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 958 | Heptan-2-ol<6-Methyl- | 0.13 | 950 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 981 | Hepten-2-one <6-Methyl-5-> | 0.38 | 984 | 0.59 | 984 | 0.63 | 984 | 0.57 | 984 |
| 1024 | Limonene | 0.45 | 1027 | 0.55 | 1028 | 0.52 | 1028 | 0.52 | 1028 |
| 1085 | Cyclohexanedione <3-Methyl-1,2-> | 0.17 | 1090 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1095 | Linalool | 0.32 | 1099 | 0.52 | 1099 | 0.56 | 1099 | 0.52 | 1099 |
| 1140 | Isocitral<Exo-> | 0.12 | 1143 | 0.16 | 1143 | 0.17 | 1143 | 0.16 | 1143 |
| 1160 | Isocitral<Z-> | 0.73 | 1162 | 0.59 | 1163 | 0.57 | 1163 | 0.55 | 1163 |
| 1177 | Isocitral<E-> | 1.41 | 1181 | 1.61 | 1181 | 1.55 | 1181 | 1.49 | 1181 |
| 1227 | Nerol | 0.46 | 1227 | 0.71 | 1229 | 0.8 | 1229 | 0.76 | 1229 |
| 1235 | Neral | 39.35 | 1242 | 41.89 | 1245 | 41.48 | 1245 | 40.91 | 1245 |
| 1249 | Geraniol | 0.77 | 1253 | 0.79 | 1256 | 0.66 | 1255 | 0.6 | 1255 |
| 1268 | Geranial | 50.76 | 1272 | 49.92 | 1276 | 50.37 | 1276 | 51.25 | 1276 |
| 1288 | Lavandulyl Acetate | 0.2 | 1278 | n.d. | n.d. | n.d. | n.d. | n.d | n.d. |
| 1290 | Tridecene <1-> | 1.31 | 1290 | n.d. | n.d. | n.d. | n.d. | n.d | n.d. |
| n.d. | Geranic acid | n.d. | n.d. | 0.14 | 1356 | 0.19 | 1356 | 0.16 | 1356 |
| 1365 | Undecenol<2E-> | 0.74 | 1371 | n.d. | n.d. | n.d. | n.d. | n.d | n.d. |
| 1389 | Elemene<Beta-> | 0.27 | 1390 | n.d. | n.d. | n.d. | n.d. | n.d | n.d. |
| 1452 | Humulene <Alpha-> | 0.67 | 1452 | 0.19 | 1452 | 0.16 | 1452 | 0.17 | 1452 |
| 1608 | Humulene Epoxide II | 0.23 | 1606 | 0.23 | 1607 | 0.21 | 1607 | 0.23 | 1607 |
| Nano-Emulsion (Replicate 1) | |||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 7 | Day 14 | Day 30 | Day 60 | Day 90 | |
| Size (nm) | 119.8 ± 0.4509 | 117.4 ± 0.7638 | 114.2 ± 1.012 | 117.5 ± 0.4163 | 138.9 ± 0.1732 | 180.4 ± 1.286 | 217.7 ± 1.900 |
| Polydispersity index | 0.217 ± 0.018 | 0.221 ± 0.005 | 0.201 ± 0.009 | 0.158 ± 0.006 | 0.119 ± 0.013 | 0.086 ± 0.012 | 0.083 ± |
| Zeta potential | −3.66 ± 0.0902 | −2.17 ± 0.148 | −2.53 ± 0.127 | −1.61 ± 0.142 | −1.31 ± 0.0702 | −8.05 ± 0.230 | −0.279 ± 0.406 |
| Nano-Emulsion (Replicate 2) | |||||||
| Day 0 | Day 1 | Day 7 | Day 14 | Day 30 | Day 60 | Day 90 | |
| Size (nm) | 113.4 ± 1.015 | 111.7 ± 0.6658 | 109.0 ± 0.3786 | 111.0 ± 0.8386 | 125.0 ± 0.4041 | 155.4 ± 1.834 | 180.5 ± 1.604 |
| Polydispersity index | 0.223 ± 0.018 | 0.209 ± 0.011 | 0.193 ± 0.006 | 0.171 ± 0.005 | 0.119 ± 0.016 | 0.091 ± 0.007 | 0.089 ± 0.026 |
| Zeta potential | −2.34 ± 0.366 | −1.44 ± 0.369 | −3.04 ± 0.215 | −1.77 ± 0.0757 | −1.63 ± 0.429 | −3.11 ± 0.527 | −0.0173 ± 0.266 |
| Nano-Emulsion (Replicate 3) | |||||||
| Day 0 | Day 1 | Day 7 | Day 14 | Day 30 | Day 60 | Day 90 | |
| Size (nm) | 115.8 ± 0.1155 | 114.2 ± 1.350 | 114.7 ± 0.1155 | 111.3 ± 0.1528 | 123.1 ± 1.015 | 155.0 ± 1.082 | 183.4 ± 1.058 |
| Polydispersity index | 0.204 ± 0.011 | 0.223 ± 0.017 | 0.226 ± 0.018 | 0.176 ± 0.008 | 0.120 ± 0.014 | 0.087 ± 0.022 | 0.074 ± 0.015 |
| Zeta potential | −2.38 ± 0.123 | −2.03 ± 0.0529 | −4.40 ± 0.0896 | −2.42 ± 0.199 | −2.36 ± 0.0252 | −3.42 ± 0.173 | −1.69 ± 0.383 |
| Average | |||||||
| Day 0 | Day 1 | Day 7 | Day 14 | Day 30 | Day 60 | Day 90 | |
| Size (nm) | 116.4 ± 2.868 | 114.4 ± 2.612 | 112.6 ± 2.820 | 113.3 ± 3.180 | 129.0 ± 7.484 | 163.6 ± 12.64 | 193.9 ± 19.96 |
| Polydispersity index | 0.215 ± 0.016 | 0.218 ± 0.012 | 0.207 ± 0.018 | 0.168 ± 0.010 | 0.119 ± 0.012 | 0.088 ± 0.013 | 0.082 ± 0.018 |
| Zeta potential | −2.80 ± 0.681 | −1.88 ± 0.390 | −3.32 ± 0.847 | −1.93 ± 0.391 | −1.77 ± 0.515 | −4.86 ± 2.41 | −0.662 ± 0.838 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.F.; Barroso, A.; Mourão, R.H.V.; Fernandes, C.P. A Low Energy Approach for the Preparation of Nano-Emulsions with a High Citral-Content Essential Oil. Molecules 2021, 26, 3666. https://doi.org/10.3390/molecules26123666
Pereira SF, Barroso A, Mourão RHV, Fernandes CP. A Low Energy Approach for the Preparation of Nano-Emulsions with a High Citral-Content Essential Oil. Molecules. 2021; 26(12):3666. https://doi.org/10.3390/molecules26123666
Chicago/Turabian StylePereira, Suelen F., Adenilson Barroso, Rosa H. V. Mourão, and Caio P. Fernandes. 2021. "A Low Energy Approach for the Preparation of Nano-Emulsions with a High Citral-Content Essential Oil" Molecules 26, no. 12: 3666. https://doi.org/10.3390/molecules26123666
APA StylePereira, S. F., Barroso, A., Mourão, R. H. V., & Fernandes, C. P. (2021). A Low Energy Approach for the Preparation of Nano-Emulsions with a High Citral-Content Essential Oil. Molecules, 26(12), 3666. https://doi.org/10.3390/molecules26123666