The Occurrence and Biological Activity of Tormentic Acid—A Review
Abstract
:1. Introduction
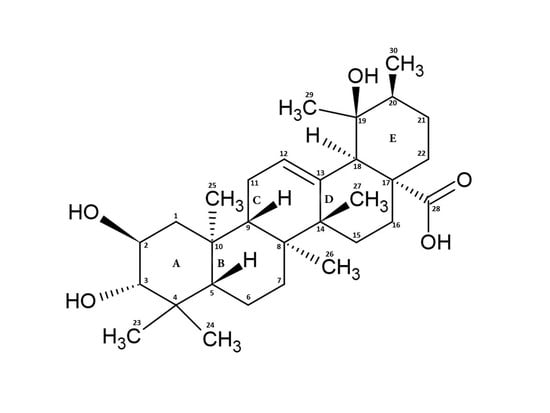
2. Structure, Function, and Occurrence of TA
3. Pharmacological Activity of TA
3.1. Anti-Inflammatory Activity
3.2. Antidiabetic Activity
3.3. Hepatoprotective Activity
3.4. Cardioprotective Activity
3.5. Anti-Cancer Activity
3.6. Anti-Osteoarthritic Activity
3.7. Antibacterial, Antifungal, Antiviral, and Antiparasitic Activity
3.8. Neuroprotective Activity
4. Derivatives of Tormentic Acid
- others, e.g., 6-methoxy-β-glucopyranosyl ester [112]; dihydrotormentic acid and methoxytormentic acid [110]; 3b-p-hydroxybenzoyloxytormentic acid [123]; (3R,19R)-methyl-3,19-dihydroxy-2-oxo-urs-12-en-28-carboxylate; (2R,19R)–methyl-2,19-dihydroxy-3-oxo-urs-12-en-28-carboxylate; (19R)-methyl-2,19-dihydroxyursa-3-oxo-1,12-dien-28-carboxylate; (2S,3R,19R)–methyl-2,3,19-trihydroxyurs-12-en-28-carboxylate; (2R,3R,19R)-2,3-bis(acetyloxy)-19-hydroxyurs-12-en-28-carboxylic acid; (2R,3R,19R)-2-acetyloxy-3,19-dihydroxyurs-12-en-28-carboxylic acid; (2R,3R,19R)-3-acetyloxy-2,19-dihydroxyurs-12-en-28-carboxylic acid; (3R,19R)–methyl-3-acetyloxy-19-hydroxy-2-oxo-urs-12-en-28-carboxylate; (2R,19R)-methyl-2-acetyloxy-19-hydroxy-3-oxo-urs-12-en-28-carboxylate; (2R,3R,19R)–methyl-2,3-bis(chloroacetyloxy)-19-hydroxy-urs-12-en-28-carboxylate; (2R,3R,19R)–methyl-2-chloroacetyloxy-3,19-dihydroxyurs-12-en-28-carboxylate; (2R,3R,19R)–methyl-3-chloroacetyloxy-2,19-dihydroxyurs-12-en-28-carboxylate [9].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombrea, A.; Scurtu, A.D.; Avram, S.; Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Dehelean, C.A.; Soica, C.; Danciu, C. Anticancer potential of betulonic acid derivatives. Int. J. Mol. Sci. 2021, 22, 3676. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, M.; Alsayari, A.; Gurusamy, N.; Louis, J.; Elbehairi, S.E.; Venkatesan, K.; Annadurai, S.; Asiri, Y.I.; Shati, A.; Saleh, K.; et al. Analgesic, Anti-Inflammatory, Cytotoxic Activity Screening and UPLC-PDA-ESI-MS Metabolites Determination of Bioactive Fractions of Kleinia pendula. Molecules 2020, 25, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, C.-C.; Ciou, J.-L.; Lin, C.-H.; Wu, J.-B.; Ho, H.-Y. Cell Suspension Culture of Eriobotrya japonica Regulates the Diabetic and Hyperlipidemic Signs of High-Fat-Fed Mice. Molecules 2013, 18, 2726–2753. [Google Scholar] [CrossRef] [Green Version]
- Akbar, E.; Malik, A. Antimicrobial Triterpenes from Debregeasia salicifolia. Nat. Prod. Lett. 2002, 16, 339–344. [Google Scholar] [CrossRef]
- De Tommasi, N.; De Simone, F.; Pizza, C.; Mahmood, N.; Moore, P.S.; Conti, C.; Orsi, N.; Stein, M.L. Constituents of Eriobotrya japonica. A Study of Their Antiviral Properties. J. Nat. Prod. 1992, 55, 1067–1073. [Google Scholar] [CrossRef]
- Hu, F.; Liao, X.; Guo, Y.; Yamaki, S.; Li, X.; Hamada, N.; Hashi, Y.; Chen, Z. Fast determination of isomeric triterpenic acids in Osmanthus fragrans (Thunb.) Lour. fruits by UHPLC coupled with triple quadrupole mass spectrometry. Food Chem. 2020, 322, 126781. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [Green Version]
- Csuk, R.; Siewert, B.; Dressel, C.; Schäfer, R. Tormentic acid derivatives: Synthesis and apoptotic activity. Eur. J. Med. Chem. 2012, 56, 237–245. [Google Scholar] [CrossRef]
- Blundell, R.; Azzopardi, J.; Briffa, J.; Rasul, A.; de la Cruz, C.V.; Shah, M.A. Analysis of pentaterpenoids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 457–475. [Google Scholar]
- Jang, D.-S.; Kim, J.-M.; Lee, G.-Y.; Kim, J.-H.; Kim, J.-S. Ursane-Type Triterpenoids from the Aerial Parts of Potentilla discolor. J. Appl. Biol. Chem. 2006, 49, 48–50. [Google Scholar]
- Jeandet, P. Phytoalexins: Current Progress and Future Prospects. Molecules 2015, 20, 2770–2774. [Google Scholar] [CrossRef]
- González-Coloma, A.; López-Balboa, C.; Santana, O.; Reina, M.; Fraga, B.M. Triterpene-based plant defenses. Phytochem. Rev. 2011, 10, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Hirai, N.; Sugie, M.; Wada, M.; Lahlou, E.H.; Kamo, T.; Yoshida, R.; Tsuda, M.; Ohigashi, H. Triterpene Phytoalexins from Strawberry Fruit. Biosci. Biotechnol. Biochem. 2000, 64, 1707–1712. [Google Scholar] [CrossRef]
- Park, K.J.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Bioactive triterpenoids from twigs of Betula schmidtii. Bioorg. Chem. 2018, 77, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.D.G.; Simões, M.; Lúcio, K.A.; Oliveira, R.R.; Kaplan, M.A.C.; Gattass, C.R. Natural triterpenoids from Cecropia lyratiloba are cytotoxic to both sensitive and multidrug resistant leukemia cell lines. Bioorg. Med. Chem. 2007, 15, 7355–7360. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Oh, S.-R.; Ahn, K.-S.; Kim, J.-G.; Lee, H.-K. Structure determination of a new lupane-type triterpene, tiarellic acid, isolated from Tiarella polyphylla. Arch. Pharmacal Res. 2002, 25, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, B.; Wang, Y.; Bao, F.; Li, H.; Chen, L. Chemical constituents of Anchusa italica Retz. and their protective effects on cardiomyocytes injured by hypoxia/reoxygenation. Phytochem. Lett. 2020, 38, 155–160. [Google Scholar] [CrossRef]
- Ibrahim, M.; Sowemimo, A.; Venables, L.; Koorbanally, N.; Awolola, G.; Sofidiya, M.; Odukoya, O.; Koekemoer, T.; Van De Venter, M. Biological evaluation of phytoconstituents from Markhamia tomentosa ethanolic leaf extract. S. Afr. J. Bot. 2018, 115, 31–36. [Google Scholar] [CrossRef]
- Dagli, M.; Sarikahya, N.B.; Nalbantsoy, A.; Kirmizigul, S. Comparative Phytochemical Screening and Cytotoxic Efficacy of Endemic Cephalaria tuteliana. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, C.; Li, Y.; Ke, C.-Q.; Lin, G.; Xie, H.; Yao, S.; Ye, Y. The first phytochemical investigation of Rhododendron websterianum: Triterpenoids and their cytotoxic activity. Phytochem. Lett. 2018, 25, 43–46. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Gao, H.-X.; Zhang, Z.; He, Z.; He, Q.; Jia, L.-R.; Zeng, W.-C. Inhibitory effects of Ligustrum robustum (Rxob.) Blume extract on α-amylase and α-glucosidase. J. Funct. Foods 2015, 19, 204–213. [Google Scholar] [CrossRef]
- Saimaru, H.; Orihara, Y.; Tansakul, P.; Kang, Y.-H.; Shibuya, M.; Ebizuka, Y. Production of Triterpene Acids by Cell Suspension Cultures of Olea europaea. Chem. Pharm. Bull. 2007, 55, 784–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, D.S.; Kim, J.M.; Kim, J.-H.; Kim, J.S. 24-nor-Ursane Type Triterpenoids from the Stems of Rumex japonicus. Chem. Pharm. Bull. 2005, 53, 1594–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, C.; Carvalho, C.; Serra, N.; Souza, I.; Costa, J.; Santana, A. Allelopathic effects of Cecropia pachystachya Trecul on germination and seedling growth of Lactuca sativa. Allelopath. J. 2017, 42, 265–280. [Google Scholar] [CrossRef]
- Olafsdottir, E.S.; Omarsdottir, S.; Jaroszewski, J.W. Constituents of three Icelandic Alchemilla species. Biochem. Syst. Ecol. 2001, 29, 959–962. [Google Scholar] [CrossRef]
- Nam, J.H.; Jung, H.J.; Tapondjou, L.A.; Lee, K.T.; Choi, J.; Kim, W.B.; Park, H.J. The anti-hyperlipidemic effect and constituents of the 19α- hydroxyursane-type triterpenoid fraction obtained from the leaves of Rubus crataegifolius. Nat. Prod. Sci. 2007, 13, 152–159. [Google Scholar]
- Dimitrova, L.; Zaharieva, M.M.; Popova, M.; Kostadinova, N.; Tsvetkova, I.; Bankova, V.; Najdenski, H. Antimicrobial and antioxidant potential of different solvent extracts of the medicinal plant Geum urbanum L. Chem. Cent. J. 2017, 11, 113. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.T.; Nguyen, D.H.; Lee, B.M.; Seong, S.H.; Choi, J.S.; Min, B.S.; Woo, M.H. PTP1B inhibitory and cytotoxic activities of triterpenoids from the aerial parts of Agrimonia pilosa. Med. Chem. Res. 2017, 26, 2870–2878. [Google Scholar] [CrossRef]
- Chu, W.; Gao, P.; Li, L. Chemical constituents from the leaves of Crataegus pinnatifida Bge. Biochem. Syst. Ecol. 2019, 86, 103923. [Google Scholar] [CrossRef]
- Jian, T.; Ding, X.; Li, J.; Wu, Y.; Ren, B.; Li, J.; Lv, H.; Chen, J.; Li, W. Triterpene Acids of Loquat Leaf Improve Inflammation in Cigarette Smoking Induced COPD by Regulating AMPK/Nrf2 and NFκB Pathways. Nutrients 2020, 12, 657. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.J.; Nam, J.H.; Choi, J.; Lee, K.T.; Park, H.J. 19α-hydroxyursane-type triterpenoids: Antinociceptive anti-inflammatory principles of the roots of Rosa rugosa. Biol. Pharm. Bull. 2005, 28, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Liang, J.; Xie, H.; Wei, X. Norsesquiterpenoids and triterpenoids from strawberry cv. Falandi. Food Chem. 2016, 203, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Hajinisa; Nurulla, A.; Li, G.; Ma, G.; Cheng, Y. Chemical constituents of Cydonia oblonga seeds and their PTP1B inhibitory effects. Acta Pharm. Sin. 2019, 54, 510–513. [Google Scholar] [CrossRef]
- Shih, C.-C.; Lin, C.-H.; Wu, J.-B. Eriobotrya japonica improves hyperlipidemia and reverses insulin resistance in high-fat-fed mice. Phytother. Res. 2010, 24, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Ullah, R.; Alqahtani, A.S.; Alsaid, M.S.; Husseiny, H.A.; Al Meanazel, O.T.R. Hepatoprotective Effect of Eriobotrya japonica Leaf Extract and Its Various Fractions against Carbon Tetra Chloride Induced Hepatotoxicity in Rats. Evid. Based Complement. Altern. Med. 2018, 2018, 3782768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-T.; Huang, S.-S.; Lin, S.-S.; Amagaya, S.; Ho, H.-Y.; Hou, W.-C.; Shie, P.-H.; Wu, J.-B.; Huang, G.-J. Anti-inflammatory activities of tormentic acid from suspension cells of Eriobotrya Japonica ex vivo and in vivo. Food Chem. 2011, 127, 1131–1137. [Google Scholar] [CrossRef]
- Taniguchi, S.; Imayoshi, Y.; Kobayashi, E.; Takamatsu, Y.; Ito, H.; Hatano, T.; Sakagami, H.; Tokuda, H.; Nishino, H.; Sugita, D.; et al. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry 2002, 59, 315–323. [Google Scholar] [CrossRef]
- Li, H.-H.; Zeng, B.-Y.; Chang, Q.; Su, M.-H.; Yao, D.-H.; Wang, W.; Xu, J. Anti-hepatocellular carcinoma activity of tormentic acid derived from suspension cells of Eriobotrya japonica (Thunb.) Lindl. Plant. Cell Tissue Organ. Cult. (PCTOC) 2017, 130, 427–433. [Google Scholar] [CrossRef]
- Ho, H.Y.; Liang, K.Y.; Lin, W.C.; Kitanaka, S.; Wu, J. Bin Regulation and improvement of triterpene formation in plant cultured cells of Eriobotrya japonica Lindl. J. Biosci. Bioeng. 2010, 110, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Julio, L.F.; Martín, L.; Muñoz, R.; Mainar, A.M.; Urieta, J.S.; Sanz, J.; Burillo, J.; González-Coloma, A. Comparative chemistry and insect antifeedant effects of conventional (Clevenger and Soxhlet) and supercritical extracts (CO2) of two Lavandula luisieri populations. Ind. Crop. Prod. 2014, 58, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Higashihara, H.; Ukiya, M.; Watanabe, K.; Kimura, Y.; Hasegawa, J.I.; Nishino, H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004, 68, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Al-Qudah, M.A.; Tashtoush, H.I.; Khlaifat, E.F.; Ibrahim, S.O.; Saleh, A.M.; Al-Jaber, H.I.; Abu Zarga, M.H.; Abu Orabi, S.T. Chemical constituents of the aerial parts of Salvia judaica Boiss. from Jordan. Nat. Prod. Res. 2019, 34, 2981–2985. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Song, C.; Suo, Y.; You, J. A sensitive and efficient method for simultaneous trace detection and identification of triterpene acids and its application to pharmacokinetic study. Talanta 2012, 98, 101–111. [Google Scholar] [CrossRef]
- Wang, J.W.; Xia, Z.; Chu, J.; Tan, R. Simultaneous production of anthocyanin and triterpenoids in suspension cultures of Perilla frutescens. Enzym. Microb. Technol. 2004, 34, 651–656. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Z.; Tan, R. High-performance liquid chromatographic analysis of bioactive triterpenes in Perilla frutescens. J. Pharm. Biomed. Anal. 2003, 32, 1175–1179. [Google Scholar] [CrossRef]
- Fujita, T.; Funayoshi, A.; Nakayama, M. A phenylpropanoid glucoside from Perilla frutescens. Phytochemistry 1994, 37, 543–546. [Google Scholar] [CrossRef]
- Ojinnaka, C.M.; Okogun, J.I.; Okorie, D.A. Triterpene acids from Myrianthus arboreus. Phytochemistry 1980, 19, 2482–2483. [Google Scholar] [CrossRef]
- Ojinnaka, C.M.; Kenne, L. Studies on Nigerian Medicinal Plants: Components of the Stems of Myrianthus arboreus. J. Nat. Prod. 1985, 48, 1002–1003. [Google Scholar] [CrossRef]
- Ngounou, F.; Lontsi, D.; Sondengam, B. A pentacyclic triterpene diacid from Myrianthus arboreus. Phytochemistry 1988, 27, 2287–2289. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, F.; Hu, J.; Liang, S.; Zhang, Y.; Du, G.; Zhang, C.; Cheng, Y. Antibacterial Lignans and Triterpenoids from Rostellularia procumbens. Planta Med. 2007, 73, 1596–1599. [Google Scholar] [CrossRef]
- Gopalsamy, N.; Vargas, D.; Guého, J.; Ricaud, C.; Hostettmann, K. Saponins from leaves of Aphloia theiformis. Phytochemistry 1988, 27, 3593–3595. [Google Scholar] [CrossRef]
- Grauzdytė, D.; Pukalskas, A.; El Kalamouni, C.; Venskutonis, P.R. Mangiferin Rich Products from Aphloia theiformis (Vahl) Benn Leaves: Extraction, Fractionation, Phytochemical Characterization, and Antioxidant Properties. Molecules 2020, 25, 2081. [Google Scholar] [CrossRef]
- Nchu, F.; Aderogba, M.; Mdee, L.; Eloff, J. Isolation of anti-Candida albicans compounds from Markhamia obtusifolia (Baker) Sprague (Bignoniaceae). S. Afr. J. Bot. 2010, 76, 54–57. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, B.K.; Hamed, A.N.E.; Samy, M.N.; Mostafa, E.M.; Wanas, A.S.; Radwan, M.M.; Elsohly, M.A.; Kamel, M.S. Phytochemical composition and antimicrobial properties of Markhamia platycalyx (Baker) Sprague leaf. Trop. J. Pharm. Res. 2019, 18, 2623–2631. [Google Scholar]
- Yang, M.-H.; Blunden, G.; O’Neill, M.J.; Lewis, J.A. Tormentic Acid and 2α-Hydroxyursolic Acid from Arnebia euchroma. Planta Med. 1992, 58, 227. [Google Scholar] [CrossRef]
- Deng, X.; Liu, X.; Zhu, D.; Wang, Q. A New Triterpenoid Saponin from Psammosilene tunicoides. Chin. J. Nat. Med. 2009, 7, 101–104. [Google Scholar] [CrossRef]
- Yamagishi, T.; Zhang, D.-C.; Chang, J.-J.; McPhail, D.R.; McPhail, A.T.; Lee, K.-H. The cytotoxic principles of Hyptis capitata and the structures of the new triterpenes hyptatic acid-A and -B. Phytochemistry 1988, 27, 3213–3216. [Google Scholar] [CrossRef]
- Jintao, X.; Shasha, Y.; Jincai, W.; Chunyan, L.; Mengya, Y.; Yongli, S. Network Pharmacological Screening of the Active Ingredients and Hypoglycemic Effect of Isodon rubescens in the Treatment of Diabetes. Planta Med. 2020, 86, 556–564. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.S.; de Figueiredo, P.T.R.; Veloso, C.A.G.; Rodrigues, G.C.S.; dos Maia, S.M.; Monteiro, A.F.M.; Rodrigues, V.S.; Castelo-Branco, A.P.O.T.; de Agra, F.M.; Filho, R.B.; et al. A new labdane diterpene from the aerial segments of Leptohyptis macrostachys (L’Hérit.) Harley & J.F.B. Pastore. Phytochem. Lett. 2021, 43, 117–122. [Google Scholar]
- Dzoyem, J.P.; Nganteng, D.N.D.; Melong, R.; Wafo, P.; Ngadjui, B.; Allémann, E.; Delie, F. Bioguided identification of pentacyclic triterpenoids as anti-inflammatory bioactive constituents of Ocimum gratissimum extract. J. Ethnopharmacol. 2021, 268, 113637. [Google Scholar] [CrossRef] [PubMed]
- Ngezahayo, J.; Pottier, L.; Ribeiro, S.O.; Delporte, C.; Fontaine, V.; Hari, L.; Stévigny, C.; Duez, P. Plastotoma rotundifolium aerial tissue extract has antibacterial activities. Ind. Crop. Prod. 2016, 86, 301–310. [Google Scholar] [CrossRef]
- Lu, X.; Xu, W.; Shen, J.; Han, G. [Chemical studies on Campylotropis hirtella (Franch. Schindl.)]. China J. Chin. Mater. Med. 1997, 22, 680–682. [Google Scholar]
- Sandjo, L.P.; Tchoukoua, A.; Ntede, H.N.; Yemloul, M.; Perspicace, E.; Keumedjio, F.; Couty, F.; Kirsch, G.; Ngadjui, B.T. New Nortriterpenoid and Ceramides from Stems and Leaves of Cultivated Triumfetta cordifolia A Rich (Tiliaceae). J. Am. Oil Chem. Soc. 2010, 87, 1167–1177. [Google Scholar] [CrossRef]
- Verardo, G.; Gorassini, A.; Fraternale, D. New triterpenic acids produced in callus culture from fruit pulp of Acca sellowiana (O. Berg) Burret. Food Res. Int. 2019, 119, 596–604. [Google Scholar] [CrossRef]
- Mashezha, R.; Mombeshora, M.; Mukanganyama, S. Effects of Tormentic Acid and the Extracts from Callistemon citrinus on the Production of Extracellular Proteases by Staphylococcus aureus. Biochem. Res. Int. 2020, 2020, 6926320. [Google Scholar] [CrossRef] [PubMed]
- Fayek, N.M.; Farag, M.A.; Saber, F. Metabolome classification via GC/MS and UHPLC/MS of olive fruit varieties grown in Egypt reveal pickling process impact on their composition. Food Chem. 2021, 339, 127861. [Google Scholar] [CrossRef] [PubMed]
- Palme, E.; Bilia, A.R.; Morelli, I. Flavonols and isoflavones from Cotoneaster simonsii. Phytochemistry 1996, 42, 903–905. [Google Scholar] [CrossRef]
- Chen, J.; Wu, R.; Hsiao, J.; Chen, L.; Zhu, T.; Kuo, Y.; Sung, P.; Cheng, M.; Chang, T.-C. A new triterpenoid and bioactive constituents of Eriobotrya deflexa f. buisanensis. Chem. Nat. Compd. 2019, 55, 74–78. [Google Scholar] [CrossRef]
- Hong, Y.; Qiao, Y.; Lin, S.; Jiang, Y.; Chen, F. Characterization of antioxidant compounds in Eriobotrya fragrans Champ leaf. Sci. Hortic. 2008, 118, 288–292. [Google Scholar] [CrossRef]
- Xu, H.-X.; Zeng, F.-Q.; Wan, M.; Sim, K.-Y. Anti-HIV Triterpene Acids from Geum japonicum. J. Nat. Prod. 1996, 59, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, L.; Catalano, S.; Miarelli, C.; Cioni, P.L.; Campeol, E. In vitro Antimicrobial Activity of Extracts and Isolated Constituents of Geum rivale. Phyther. Res. 2000, 14, 561–563. [Google Scholar] [CrossRef]
- Verardo, G.; Gorassini, A.; Ricci, D.; Fraternale, D. High Triterpenic Acids Production in Callus Cultures from Fruit Pulp of Two Apple Varieties. Phytochem. Anal. 2016, 28, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Tommasi, N.; Rastrelli, L.; Cumanda, J.; Speranza, G.; Pizza, C. Aryl and triterpenic glycosides from Margyricarpus setosus. Phytochemistry 1996, 42, 163–167. [Google Scholar] [CrossRef]
- Li, Q.; Hui, J.; Shang, D.; Wu, L.; Ma, X. Investigation of the Chemical Constituents of the Roots of Potentilla anserina L. in Tibet. J. Chin. Pharm. Sci. 2003, 55, 179–184. [Google Scholar]
- Zhang, L.; Le Jian, L.; Li, J.Y.; Jin, X.; Li, L.Z.; Zhang, Y.L.; Gong, H.Y.; Cui, Y. Possible involvement of alpha B-crystallin in the cardioprotective effect of n-butanol extract of Potentilla anserina L. on myocardial ischemia/reperfusion injury in rat. Phytomedicine 2019, 55, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, S.; Huang, R.; Tan, S.; Liang, S.; Wu, X.; Zhuo, L.; Huang, Q. Protective effect of tormentic acid from Potentilla chinensis against lipopolysaccharide/D-galactosamine induced fulminant hepatic failure in mice. Int. Immunopharmacol. 2014, 19, 365–372. [Google Scholar] [CrossRef]
- Kumar, D.; Ghosh, R.; Pal, B.C. α-Glucosidase inhibitory terpenoids from Potentilla fulgens and their quantitative estimation by validated HPLC method. J. Funct. Foods 2013, 5, 1135–1141. [Google Scholar] [CrossRef]
- Villar, A.; Paya, M.; Hortiguela, M.D.; Cortes, D. Tormentic acid, a new hypoglycemia agent from Poterium ancistroides. Planta Med. 1986, 52, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Ivorra, M.; Payá, M.; Villar, A. Hypoglycemic and Insulin Release Effects of Tormentic Acid: A New Hypoglycemic Natural Product. Planta Med. 1988, 54, 282–286. [Google Scholar] [CrossRef]
- Jovel, E.M.; Zhou, X.L.; Ming, D.S.; Wahbe, T.R.; Towers, G.H.N. Bioactivity-guided isolation of the active compounds from Rosa nutkana and quantitative analysis of ascorbic acid by HPLC. Can. J. Physiol. Pharmacol. 2007, 85, 865–871. [Google Scholar] [CrossRef]
- Li, Q.-J.; Nan, Y.; Qin, J.-J.; Yang, Y.; Hao, X.-J.; Yang, X.-S. Chemical constituents from medical and edible plants of Rosa roxburghii. Zhongguo Zhong Yao Za Zhi 2016, 41, 451–455. [Google Scholar]
- Jiang, C.-X.; Mao, J.-H.; Wang, W.-Y.; Cheng, W.-L.; Cheng, K.-J. Chemical constituents of traditional She medicine Rubi Radix et Rhizoma. Chin. Tradit. Herb. Drugs 2016, 47, 3370–3373. [Google Scholar]
- Kim, S.; Oh, S.; Noh, H.B.; Ji, S.; Lee, S.H.; Koo, J.M.; Choi, C.W.; Jhun, H.P. In Vitro Antioxidant and Anti-Propionibacterium acnes Activities of Cold Water, Hot Water, and Methanol Extracts, and Their Respective Ethyl Acetate Fractions, from Sanguisorba officinalis L. Roots. Molecules 2018, 23, 3001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loizzo, M.R.; Bonesi, M.; Passalacqua, N.G.; Saab, A.; Menichini, F.; Tundis, R. Antiproliferative activities on renal, prostate and melanoma cancer cell lines of Sarcopoterium spinosum aerial parts and its major constituent tormentic acid. Anti-Cancer Agents Med. Chem. 2013, 13, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, S.; Wu, X.; Yu, Y.; Yue, Z.; Liu, B.; Lin, S.; Zhu, C.; Yang, Y.; Shi, J. Non-anthraquinones constituents from the roots of Knoxia valerianoides. China J. Chin. Mater. Medica 2012, 37, 2092–2099. [Google Scholar]
- Fru, C.G.; Sandjo, L.P.; Kuete, V.; Liermann, J.; Schollmeyer, D.; Yeboah, S.O.; Mapitse, R.; Abegaz, B.M.; Ngadjui, B.T.; Opatz, T. Omphalocarpoidone, a new lanostane-type furano-spiro-γ-lactone from the wood of Tridesmostemon omphalocarpoides Engl. (Sapotaceae). Phytochem. Lett. 2013, 6, 676–680. [Google Scholar] [CrossRef]
- Chen, J.; Ni, L.; Zhang, Y.; Zhu, Y.; Huang, W.; Zou, S. Chemical Constituents and Their Activities from the Twigs of Euscaphis konishii Hayata. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Lontsi, D.; Ngounou, N.F.; Tapondjou, A.L.; Sondengam, B.L.; Bodo, B.; Martin, M.-T. An E-ring γ-lactone pentacyclic triterpene from Myrianthus serratus. Phytochemistry 1998, 49, 2473–2476. [Google Scholar] [CrossRef]
- Torres-Santos, E.; Lopes, D.; Oliveira, R.R.; Carauta, J.; Falcao, C.B.; Kaplan, M.; Rossi-Bergmann, B. Antileishmanial activity of isolated triterpenoids from Pourouma guianensis. Phytomedicine 2004, 11, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Bhowmik, J.; Ghosh, R.; Das, M.C.; Sandhu, P.; Kumari, M.; Acharjee, S.; Daware, A.V.; Akhter, Y.; Banerjee, B.; et al. The anti-biofilm potential of triterpenoids isolated from Sarcochlamys pulcherrima (Roxb.) Gaud. Microb. Pathog. 2020, 139, 103901. [Google Scholar] [CrossRef] [PubMed]
- Bortalanza, L.B.; Ferreira, J.; Hess, S.C.; Monache, F.D.; Yunes, R.A.; Calixto, J.B. Anti-allodynic action of the tormentic acid, a triterpene isolated from plant, against neuropathic and inflammatory persistent pain in mice. Eur. J. Pharmacol. 2002, 453, 203–208. [Google Scholar] [CrossRef]
- Fogo, A.S.; Antonioli, E.; Calixto, J.B.; Campos, A.H. Tormentic acid reduces vascular smooth muscle cell proliferation and survival. Eur. J. Pharmacol. 2009, 615, 50–54. [Google Scholar] [CrossRef]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Neuroprotective effect of tormentic acid against memory impairment and neuro-inflammation in an Alzheimer’s disease mouse model. Mol. Med. Rep. 2020, 22, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, N.; Jin, G.; Xue, L. Tormentic acid induces anticancer effects in cisplatin-resistant human cervical cancer cells mediated via cell cycle arrest, ROS production, and targeting mTOR/PI3K/AKT signalling pathway. J. BU ON 2020, 25, 74–79. [Google Scholar]
- Yang, Y.; Wang, Y.; Zhao, M.; Jia, H.; Li, B.; Xing, D. Tormentic acid inhibits IL-1β-induced chondrocyte apoptosis by activating the PI3K/Akt signaling pathway. Mol. Med. Rep. 2018, 17, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Wang, Y.; Zhao, M.; Jia, H.; Li, B.; Xing, D. Tormentic Acid Inhibits IL-1β-Induced Inflammatory Response in Human Osteoarthritic Chondrocytes. Inflammation 2016, 39, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Wang, Y.; Zhang, Q. Tormentic acid reduces inflammation in BV-2 microglia by activating the liver X receptor alpha. Neuroscience 2015, 287, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Sun, G.-Y.; Zhang, Y.; He, J.-J.; Zheng, S.; Lin, J.-N. Tormentic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NF-κB signaling pathway. Mol. Med. Rep. 2016, 14, 3559–3564. [Google Scholar] [CrossRef] [Green Version]
- An, H.-J.; Kim, I.-T.; Park, H.-J.; Kim, H.-M.; Choi, J.-H.; Lee, K.-T. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-α expression through inactivation of the nuclear factor-κb pathway in RAW 264.7 macrophages. Int. Immunopharmacol. 2011, 11, 504–510. [Google Scholar] [CrossRef]
- Jian, C.X.; Li, M.Z.; Zheng, W.Y.; He, Y.; Ren, Y.; Wu, Z.M.; Fan, Q.S.; Hu, Y.H.; Li, C.J. Tormentic acid inhibits LPS-induced inflammatory response in human gingival fibroblasts via inhibition of TLR4-mediated NF-κB and MAPK signalling pathway. Arch. Oral Biol. 2015, 60, 1327–1332. [Google Scholar] [CrossRef]
- Ivorra, M.D.; Payá, M.; Villar, A. Effect of tormentic acid on insulin secretion in isolated rat islets of langerhans. Phytother. Res. 1989, 3, 145–147. [Google Scholar] [CrossRef]
- Wu, J.-B.; Kuo, Y.-H.; Lin, C.-H.; Ho, H.-Y.; Shih, C.-C. Tormentic Acid, a Major Component of Suspension Cells of Eriobotrya japonica, Suppresses High-Fat Diet-Induced Diabetes and Hyperlipidemia by Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation. J. Agric. Food Chem. 2014, 62, 10717–10726. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Sonam, T.; Shimizu, K. The Potential of Triterpenoids from Loquat Leaves (Eriobotrya japonica) for Prevention and Treatment of Skin Disorder. Int. J. Mol. Sci. 2017, 18, 1030. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.-P.; Huang, S.-S.; Matsuda, Y.; Saito, H.; Uramaru, N.; Ho, H.-Y.; Wu, J.-B.; Huang, G.-J. Protective Effects of Tormentic Acid, a Major Component of Suspension Cultures of Eriobotrya japonica Cells, on Acetaminophen-Induced Hepatotoxicity in Mice. Molecules 2017, 22, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Li, Y.; Zhang, X.; Wei, Y.; Wen, S.; Lu, Z.; Huang, Q.; Wei, J. Tormentic acid inhibits hepatic stellate cells activation via blocking PI3K/Akt/mTOR and NF-κB signalling pathways. Cell Biochem. Funct. 2021, 39, 77–87. [Google Scholar] [CrossRef]
- Shi, C.; Li, Z.; Wu, Y.; Li, X.; Li, Y.; Wei, J.; Li, J.Y.; Zhang, Y.; Li, L. Euscaphic acid and Tormentic acid protect vascular endothelial cells against hypoxia-induced apoptosis via PI3K/AKT or ERK 1/2 signaling pathway. Life Sci. 2020, 252, 117666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-T.; Yang, L.; Jiang, J.-G. Tormentic acid in foods exerts anti-proliferation efficacy through inducing apoptosis and cell cycle arrest. J. Funct. Foods 2015, 19, 575–583. [Google Scholar] [CrossRef]
- Zhao, Q.; Ye, J.; Wei, N.; Fong, C.; Dong, X. Protection against MPP+-induced neurotoxicity in SH-SY5Y cells by tormentic acid via the activation of PI3-K/Akt/GSK3β pathway. Neurochem. Int. 2016, 97, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef]
- Kim, I.-T.; Ryu, S.; Shin, J.-S.; Choi, J.-H.; Park, H.-J.; Lee, K.-T. Euscaphic acid isolated from roots of Rosa rugosa inhibits LPS-induced inflammatory responses via TLR4-mediated NF-κB inactivation in RAW 264.7 macrophages. J. Cell. Biochem. 2012, 113, 1936–1946. [Google Scholar] [CrossRef]
- Fang, J.-M.; Wang, K.-C.; Cheng, Y.-S. Steroids and triterpenoids from Rosa laevigata. Phytochemistry 1991, 30, 3383–3387. [Google Scholar] [CrossRef]
- Dai, W.; Dong, P.; Liu, J.; Gao, Y.; Hu, Y.; Lin, H.; Song, Y.; Mei, Q. Euscaphic acid inhibits proliferation and promotes apoptosis of nasopharyngeal carcinoma cells by silencing the PI3K/AKT/mTOR signaling pathway. Am. J. Transl. Res. 2019, 11, 2090–2098. [Google Scholar]
- Delgado, G.; Hernández, J.; Pereda-Miranda, R. Triterpenoid acids from Cunila lythrifolia. Phytochemistry 1989, 28, 1483–1485. [Google Scholar] [CrossRef]
- Rocha, G.D.G.; Simoes, M.; Oliveira, R.R.; Kaplan, M.A.C.; Gattass, C.R. 3β-acetyl tormentic acid induces apoptosis of resistant leukemia cells independently of P-gp/ABCB1 activity or expression. Investig. New Drugs 2010, 30, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.D.G.; Simoes, M.; Oliveira, R.R.; Kaplan, M.A.C.; Gattass, C.R. Effects of 3β-Acethyl Tormentic Acid (3ATA) on ABCC Proteins Activity. Int. J. Mol. Sci. 2012, 13, 6757–6771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, G.D.G.; Oliveira, R.R.; Kaplan, M.A.C.; Gattass, C.R. 3β-Acetyl tormentic acid reverts MRP1/ABCC1 mediated cancer resistance through modulation of intracellular levels of GSH and inhibition of GST activity. Eur. J. Pharmacol. 2014, 741, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Beirith, A.; Santos, A.R.S.; Calixto, J.B.; Hess, S.C.; Messana, I.; Ferrari, F.; Yunes, R.A. Study of the Antinociceptive Action of the Ethanolic Extract and the Triterpene 24-Hydroxytormentic Acid Isolated from the Stem Bark of Ocotea suaveolens. Planta Med. 1999, 65, 050–055. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Akazawa, H.; Tabata, K.; Manosroi, A.; Manosroi, J.; Suzuki, T.; Akihisa, T. 3-O-(E)-p-coumaroyl tormentic acid from Eriobotrya japonica leaves induces caspase-dependent apoptotic cell death in human leukemia cell line. Chem. Pharm. Bull. 2011, 59, 378–381. [Google Scholar] [CrossRef] [Green Version]
- Garcez, F.R.; Garcez, W.; Martins, M.; Lopes, F. Triterpenoids, lignan and flavans from Terminalia argentea (Combretaceae). Biochem. Syst. Ecol. 2003, 31, 229–232. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Bijttebier, S.; Tuenter, E.; Custers, D.; Ortíz, O.O.; Pieters, L.; Caballero-George, C.; Apers, S.; Foubert, K. Phytochemical characterization and comparative studies of four Cecropia species collected in Panama using multivariate data analysis. Sci. Rep. 2019, 9, 1763. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Choi, S.-H.; Lee, I.-S.; Kim, Y.; An, E.-J.; Jang, H.-J. Anti-inflammatory effect of Rosa laevigata extract on in vitro and in vivo model of allergic asthma via the suppression of IgE and related cytokines. Mol. Cell. Toxicol. 2020, 16, 119–127. [Google Scholar] [CrossRef]
- Tanaka, J.C.A.; Vidotti, G.J.; Da Silva, C.C. A New tormentic acid derivative from Luehea divaricata Mart. (Tiliaceae). J. Braz. Chem. Soc. 2003, 14, 475–478. [Google Scholar] [CrossRef]

| Plant Family | Species and Organ Investigated | Extraction Solvent | Ref. |
|---|---|---|---|
| Acanthaceae | Rostellularia procumbens (L.) Nees [Justicia procumbens L.] Whole plant | 80% Ethanol | [51] |
| Aphloiaceae | Aphloia theiformis (Vahl) Benn. Leaves | Methanol | [52] |
| Aphloiaceae | Aphloia theiformis (Vahl) Benn. Leaves | 70% Ethanol | [53] |
| Betulaceae | Betula schmidtii Regel Twigs | 80% Methanol | [15] |
| Bignoniaceae | Markhamia obtusifolia (Baker) Sprague Leaves | Acetone | [54] |
| Bignoniaceae | Markhamia platycalyx (Baker) Sprague [Markhamia lutea (Benth.) K.Schum.] Leaves | 95% Ethanol | [55] |
| Bignoniaceae | Markhamia tomentosa (Benth) K. Schum ex Engl. Leaves | Ethanol | [19] |
| Boraginaceae | Anchusa italica Retz. [Anchusa azurea Mill.] Aerial parts | 75% Ethanol | [18] |
| Boraginaceae | Arnebia euchroma (Royle) I.M.Johnst. Roots | Methanol | [56] |
| Caprifoliaceae | Cephalaria tuteliana Kuș & Göktürk Not specified | Methanol | [20] |
| Caryophyllaceae | Psammosilene tunicoides W.C. Wu & C. Y. Wu. Roots | 80% Ethanol | [57] |
| Compositae | Kleinia pendula (Forssk.) DC. Fresh aerial parts | Methanol | [3] |
| Ericaceae | Rhododendron websterianum Rehder & E.H. Wilson Fruits | 95% Ethanol | [21] |
| Lamiaceae | Hyptis capitata Jacq. Leaves and stems | Methanol | [58] |
| Lamiaceae | Isodon rubescens (Hemsl.) H.Hara Whole plant | - | [59] |
| Lamiaceae | Lavandula luisieri (Rozeira) Riv.-Mart. [Lavandula stoechas subsp. luisieri (Rozeira) Rozeira] Flowering plant | Ethanol | [41] |
| Lamiaceae | Leptohyptis macrostachys (L’H’erit.), Harley and J.F.B. Pastore (previously Hyptis macrostachys Benth.) Aerial parts | 95% Ethanol | [60] |
| Lamiaceae | Ocimum gratissimum L. Aerial parts | Methanol | [61] |
| Lamiaceae | Perilla frutescens L. Britton Cell culture from leaves | Methanol | [45] |
| Lamiaceae | Perilla frutescens (L.) Britton var. acuta Kudo Fresh leaves | Methanol | [47] |
| Lamiaceae | Perilla frutescens (L.) Britton Leaves | Ethanol | [42,46] |
| Lamiaceae | Platostoma rotundifolium (Briq.) A. J. Paton Aerial parts | Ethyl acetate | [62] |
| Lamiaceae | Salvia judaica Boiss. Aerial parts | Ethanol | [43] |
| Lamiaceae | Salvia miltiorrhiza Bunge Roots and aerial parts | Ethanol | [44] |
| Leguminosae | Campylotropis hirtella (Franch.) Schindl. Roots | - | [63] |
| Malvaceae | Triumfetta cordifolia A.Rich. Stems | Methylene: methanol (1:1) | [64] |
| Myrtaceae | Acca sellowiana (O.Berg) Burret Callus culture from fruit pulp | Methanol | [65] |
| Myrtaceae | Callistemon citrinus (Curtis) Skeels Leaves | Dichloromethane: Methanol (50:50, v/v) Water: Ethanol (50:50, v/v) | [66] |
| Oleaceae | Ligustrum robustum (Roxb.) Blume Not specified | 70% Methanol | [22] |
| Oleaceae | Olea europaea L. Cell-suspension cultures (callus induced from leaf stalk) | Methanol | [23] |
| Oleaceae | Olea europaea L. (varieties Manzanilo, Picual, Koroneiki, and Coratina) Fruits | Methanol | [67] |
| Oleaceae | Osmanthus fragrans Lour Fruits | Methanol | [7] |
| Polygonaceae | Rumex japonicus Houtt. Stems | 80% Ethanol | [24] |
| Rosaceae | Agrimonia pilosa Ledeb. Aerial parts | 80% Ethanol | [29] |
| Rosaceae | Alchemilla faeroensis (J. Lange) Buser Aerial parts | Ethanol | [26] |
| Rosaceae | Cotoneaster simonsii hort. ex Baker Aerial parts (leaves and twigs) | Chloroform | [68] |
| Rosaceae | Crataegus pinnatifida Bunge Leaves | 80% Ethanol | [30] |
| Rosaceae | Cydonia oblonga Mill. Seeds | Methanol | [34] |
| Rosaceae | Eriobotrya deflexa f. buisanesis [Eriobotrya deflexa (Hemsl.) Nakai.] Leaves | Methanol | [69] |
| Rosaceae | Eriobotrya fragrans Champ. ex Benth Leaves | 95% Ethanol | [70] |
| Rosaceae | Eriobotrya japonica (Thunb) Lindl. Leaves | 80% Methanol | [36] |
| Rosaceae | Eriobotrya japonica (Thunb.) Lindl. Leaves | 95% Ethanol | [31,35] |
| Rosacae | Eriobotrya japonica (Thunb.) Lindl Cell suspension culture (callus induced from leaves) | Ethanol | [37] |
| Rosaceae | Eriobotrya japonica (Thunb.) Lindl. Callus cultures induced from an axenic leaf | Ethanol | [38] |
| Rosaceae | Eriobotrya japonica (Thunb) Lindl. Cell suspension culture (obtained from immature embryos) | 95% Ethanol | [39] |
| Rosaceae | Eriobotrya japonica (Thunb.) Lindl. Cell suspension culture (callus induced from leaves) | 95% Ethanol | [4] |
| Rosaceae | Fragaria × ananassa Duch. var ‘Falandi’ Fresh fruit | 95% Ethanol | [33] |
| Rosaceae | Fragaria × ananassa Duch. var ‘Hokouwase’ Green unripe fresh fruit | Methanol | [14] |
| Rosaceae | Geum japonicum auct. [Geum macrophyllum Willd.] Whole plant | Methanol | [71] |
| Rosaceae | Geum rivale L. Flowering aerial parts | Chloroform: Methanol (9:1) | [72] |
| Rosaceae | Geum urbanum L. Roots and aerial parts | Methanol | [28] |
| Rosaceae | Malus domestica Borkh varieties “Mela Rosa Marchigiana” and “Golden Delicious” Pulp callus culture | Methanol | [73] |
| Rosaceae | Margyricarpus setosus Ruiz & Pav. [Margyricarpus pinnatus (Lam.) Kuntze] Aerial parts | Methanol | [74] |
| Rosaceae | Potentilla anserina L. Roots | - | [75] |
| Rosaceae | Potentilla anserina L. Roots | 70% Ethanol | [76] |
| Rosaceae | Potentilla chinensis Ser. Whole plant | 95% Ethanol | [77] |
| Rosaceae | Potentilla fulgens [Potentilla lineata Trevir.] Roots | Methanol | [78] |
| Rosaceae | Poterium ancistroides Desf. [Sanguisorba ancistroides (Desf.) Ces.] Aerial parts | Ethyl acetate | [79] |
| Rosaceae | Poterium ancistroides Desf. [Sanguisorba ancistroides (Desf.) Ces.] Herb | Methanol | [80] |
| Rosaceae | Rosa nutkana C.Presl Fruits | Methanol | [81] |
| Rosaceae | Rosa roxburghii | - | [82] |
| Rosaceae | Rosa rugosa Thunb. Roots | Methanol | [32] |
| Rosaceae | Rubus chingii Hu Roots and rhizomes | Ethanol | [83] |
| Rosaceae | Rubus crataegifolius Bunge Leaves | Methanol | [27] |
| Rosaceae | Sanguisorba officinalis L. Root | Cold water Hot water Methanol | [84] |
| Rosaceae | Sarcopoterium spinosum (L.) Spach. Aerial parts | - | [85] |
| Rubiaceae | Knoxia valerianoides Thorel ex Pit. [Knoxia roxburghii subsp. brunonis (Wall. ex G.Don) R.Bhattacharjee & Deb] Roots | Ethanol | [86] |
| Sapotaceae | Tridesmostemon omphalocarpoides Engl. Wood and stem bark | Dichloromethane: Methanol (1:1) | [87] |
| Saxifragaceae | Tiarella polyphylla D. Don Whole plant | Methanol | [17] |
| Staphyleaceae | Euscaphis konishii Hayata [Euscaphis japonica (Thunb.) Kanitz] Twigs | 95% Ethanol | [88] |
| Urticaceae | Cecropialyratiloba Miq. [Cecropia pachystachya Trécul.)] Roots | Methanol | [16] |
| Urticaceae | Cecropia pachystachya Trécul Roots, root bark, stem and stem bark | Ethanol | [25] |
| Urticaceae | Debregeasia salicifolia D. Don. [Debregeasia saeneb (Forssk.) Hepper & J.R.I.Wood] Stems | Methanol | [5] |
| Urticaceae | Myrianthus arboreus P.Beauv Stem bark | Methylated ethyl acetate | [50] |
| Urticaceae | Myrianthus arboreus P.Beauv Root wood | Methylated spirit | [48] |
| Urticaceae | Myrianthus arboreus P.Beauv Stems | Chloroform | [49] |
| Urticaceae | Myrianthus serratus (Trecul) Benth. Trunk wood | Ethyl acetate | [89] |
| Urticaceae | Pourouma guianensis Aubl. Leaves | Methanol | [90] |
| Urticaceae | Sarcochlamys pulcherrima (Roxb.) Gaudich. Aerial parts | Methanol | [91] |
| Vochysiaceae | Vochysia divergens Pohl. Stem bark | Ethanol | [92,93] |
| Biological Activity | Model | Ref. |
|---|---|---|
| Anti-inflammatory (anti-osteoarthritic): –decreasing the interleukin (IL)-1β-stimulated expression of MMP-3 and MMP-13; –inhibition of the IL-1β-induced expression of iNOS and COX-2, and the production of PGE2 and NO; inhibition of IL-1β-induced NF-κB activation | In vitro Human Articular Chondrocyte Culture | [97] |
| Anti-inflammatory: –inhibition of nitric oxide (NO) and prostaglandin E 2 (PGE 2) production by inhibiting iNOS and COX-2 expression; –inhibition of LPS-stimulated production of TNF-α and IL-1β; –activation of LXRα (liver X receptor α) and inhibition of LPS-induced NF-κB activation | In vitro BV2 microglial cells | [98] |
| Antioxidative and anti-inflammatory: –decreasing reactive oxygen species (ROS) generation; –inhibition of the expression of inducible nitric oxide synthase (iNOS) and NADPH oxidase (NOX); –decreasing the production of tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and IL-1β; –preventing phosphorylation of nuclear factor-κB (NF-κB) subunit p65 and degradation of NF-κB inhibitor α (IκBα) | In vitro Rat vascular smooth muscle cells (RVSMCs); | [99] |
| Anti-inflammatory: –decreasing paw edema; –increasing the activities of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in liver tissue; –attenuating the level of thiobarbituric acid reactive substances (TBARS) in the edematous paw; –decreasing the nitric oxide (NO) levels at the serum level and diminishing the serum tumor necrosis factor (TNF-α); –decreasing the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) | Ex vivo and in vivo RAW264.7 macrophages and λ-carrageenin-induced hind paw edema model in mice | [37] |
| Anti-inflammatory: –reducing the production of NO, prostaglandin E2 (PGE2), and tumor necrosis factor-α (TNF-α) induced by LPS; –suppressing the LPS-induced expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and TNF-α at the mRNA and protein levels; –decreasing DNA binding of nuclear factor kappa B(NF-kB) and nuclear translocation of the p65 and p50 subunits of NF-kB; –suppressing degradation and phosphorylation of inhibitor of kappa B-Alpha | In vitro LPS stimulated RAW264.7 cells | [100] |
| Anti-inflammatory/antinociceptive (20–30 mg/kg) | In vivo Writhing Assay; Hot-Plate Test; Carrageenan-Induced Edema in Sprague–Dawley Rats | [32] |
| Anti-inflammatory: –inhibition of the production of interleukin-6 and interleukin-8; –inhibition of TLR4 (Toll-like receptor 4) expression; –inhibition of activation of nuclear factor kappa B (NF-κB); –inhibition of activation of mitogen-activated protein kinases (MAPKs) | In vitro LPS-stimulated human gingival fibroblasts (HGFs) | [101] |
| Anti-inflammatory: –inhibition of LPS-induced NO production | In vitro | [69] |
| Anti-inflammatory: –inhibitory effect on IFN-γ secretion –inhibition of COX-1 and COX-2 –apoptosis-inducing effect | In vitro LPS-stimulated Raw 264.7 macrophage | [61] |
| –Anti-inflammatory; –Potent inhibitory effect on Epstein-Barr virus early antigen (EBV-EA) activation; –Antitumor-promoting activity (strong) | In vivo: –TPA-induced ear edema inflammation in mice; –two-stage carcinogenesis test of mouse tumor; In vitro EBV-EA activation experiment | [42] |
| –Cytotoxic activity against the HeLa cell line; –Antidiabetic activity –Inhibition of PTP1B (Protein tyrosine phosphate) | In vitro | [29] |
| Cytotoxic to sensitive and multidrug resistant leukemia cell lines; Active toward a multidrug resistant (MDR) leukemia cell line overexpressing glycoprotein-P (P-gp) | In vitro (anti-MDR activity in Lucena-1, a leukemia cell line that overexpresses P-gp and presents cross resistance to several unrelated cytotoxic drugs) | [16] |
| Cytotoxic | In vitro HCT-8, A549, P-388, L-1210 tumor cell lines | [58] |
| –Cytotoxicity in human oral tumor cell lines: human salivary gland tumor and human oral squamous cell carcinoma –Inhibition of the activation of Epstein–Barr virus early antigen (EBV-EA) | In vivo EBV genome-carrying lymphoblastoid cells In vitro human oral squamous cell carcinoma (HSC-2), human salivary gland tumor (HSG) | [38] |
| Antidiabetic and antihyperlipidemic: –Antihyperlipidemic: decreasing gene expressions of fatty acids, increasing the content of phosphorylated AMPK-α in liver and adipose tissue, inhibition of DGAT 1 expression, and decreasing the level of triglycerides in blood –Antidiabetic: down-regulation of phosphenolpyruvate carboxykinase (PEPCK), improving insulin sensitization (at 1.0 g/kg), and decreasing the expression of the hepatic and adipose 11-β-hydroxysteroid dehydroxygenase (11β-HSD1) gene | In vivo high-fat fed C57BL/6J mice | [4] |
| Hypoglycemic: decreasing the blood glucose level (at 10 mg/kg) | In vivo normoglycemic Wistar rats | [79] |
| Hypoglycemic effect (at 30 mg/kg): –decreasing glucose levels in normal rats; –increasing fasting plasma insulin levels Acute toxicity not observed (at 600 mg/kg, intraperitoneally) | In vivo normoglycemic, hyperglycemic, and streptozotocin-induced diabetic Wistar rats | [80] |
| Hypoglycemic effect: –direct stimulation of insulin secretion by pancreatic islets of Langerhans | In vitro pancreatic islets of Langerhans isolated from fed Wistar rats | [102] |
| Antidiabetic: –inhibition of alfa-glucosidase | In vitro | [78] |
| Antidiabetic and antihyperlipidemic activity: –lowering blood glucose, triglycerides, free fatty acids, leptin levels; –decreasing the area of adipocytes and ballooning degeneration of hepatocytes; –reducing visceral fat mass, reducing hepatic triacylglycerol contents; –enhancing skeletal muscular Akt phosphorylation and increasing insulin sensitivity; –decreasing blood triglycerides by down-regulation of the hepatic sterol regulatory element binding protein-1c (SREBP-1c) and apolipoprotein C-III (apo C-III) and increasing the expression of peroxisome proliferator activated receptor (PPAR)-α | In vivo C57BL/6J mice with induced type 2 diabetes and hyperlipidemia | [103] |
| Influencing the processes present in vasculoproliferative diseases (diseases related to vascular smooth muscle cell (VSMC) abnormal proliferation): –increasing apoptosis of serum-deprived A7r5 cells and inhibiting A7r5 cell proliferation; –rapid induction of significant modifications in the vascular smooth muscle cell (VSMC) phenotype; –inhibition of VSMC proliferation and VSMC cell death | In vitro Clonal rat embryonic VSMCs (A7r5) and human umbilical vein endothelial cells (HUVEC) | [93] |
| Anti-melanogenesis effect (melanin synthesis inhibitory activity with less cytotoxicity) Antibacterial activity against Propionibacterium acnes Promotion of skin collagen synthesis | In vitro Mouse melanoma cell line B16; Propionibacterium acnes (NBRC 107605) | [104] |
| Hepatoprotective (preventing fulminant hepatic failure): –blocking the NF-κB signaling pathway for anti-inflammatory response (alleviating the pro-inflammatory cytokines, e.g., TNF-α and NO/iNOS by inhibiting nuclear factor-κB activity); –inhibition of hepatic lipid peroxidation; –decreasing serum aminotransferase and total bilirubin activities; –attenuating hepatocellular apoptosis | In vivo lipopolysaccharide/d-galactosamine-induced acute hepatic failure in male C57BL/6 mice | [77] |
| Hepatoprotective: –inhibition of the production of pro-inflammatory factors such as: tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), and IL-6; –inhibition of inducible NO synthetase (iNOS) and cyclooxygenase-2 (COX-2); –inhibition of nuclear factor –κB (NF-κB) activation; –inhibition of the activation of mitogen-activated protein kinases (MAPKs); –retention of enzymes (essential for the antioxidative properties of liver): superoxidase dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) | In vivo Acetaminophen-induced hepatotoxicity in male ICR mice | [105] |
| Protective effect against liver fibrosis: –inhibition of the activation of hepatic stellate cells; –reducing aspartate aminotransferase, alanine aminotransferase, and total bilirubin activity; –inhibition of expression of collagen type I and III; alleviation of collagen-based extracellular matrix deposition; –promoting cell apoptosis via blocking of the PI3K/Akt/mTOR and NF-κB signaling pathways | In vitro Hepatic stellate cells (HSCs) stimulated with platelet-derived growth factor-BB | [106] |
| Cardioprotective (protective effects on hypoxia/reoxygenation (H/R)-induced cardiomyocyte injury) | In vitro Neonatal rat cardiomyocytes subjected to hypoxia/reoxygenation (H/R) insult | [18] |
| Anti-hypoxic (protecting vascular endothelial cells against hypoxia-induced damage via the PI3K/AKT and ERK 1/2 signaling pathway) | In vitro (EA.hy926 cells) | [107] |
| Antiproliferative: –causing apoptosis and G0/G1 phase arrest in cancer cell lines; –induction of cell cycle arrest via changing the cyclin D1 and cyclin-dependent kinase 4 mRNA expression levels; –down-regulation of the NF-kappa-B cell survival pathway and the expression level of phosphorylated ERK (extracellular signal-regulated kinase) | In vitro Cancer cell lines: human hepatoma cells HepG-2 and Bel-7402, lung cancer cell A549, breast cancer cell MCF-7 Normal LO2 cell line | [108] |
| Antiproliferative | In vitro | [85] |
| Anti-cancer (anti-hepatocellular carcinoma activity): –decreasing cell viability, colony formation, and cell migration; –induction of apoptosis; –changing the levels of caspase-3 and poly ADP-ribose polymerase expression | In vitro Hepatocellular carcinoma cells (HepG2, Bel-7405, Sk-hep-1) | [39] |
| Anti-cancer: –induction of cell cycle arrest; –enhancement of ROS production; –targeting the mTOR/PI3K/AKT signaling pathway in cisplatin-resistant human cervical cancer cells | In vitro Cisplatin-resistant human cervical cancer cells (HeLa cells) | [95] |
| Anti-osteoarthritic (inhibition of IL-1β-induced chondrocyte apoptosis by activation of the PI3K/Akt signaling pathway): –inhibition of IL-1β induced cytotoxicity and apoptosis in chondrocytes; –increasing B-cell lymphoma (Bcl)-2 expression; –decreasing capsase-3 activity and Bax expression; –increasing the expression of p-PI3K and p-Akt in IL-1β-induced chondrocytes | In vitro IL-1β-treated human osteoarthritic chondrocytes | [96] |
| Antinociceptive (anti-allodynic) | In vivo two models of chronic pain (neuropathic pain and inflammatory pain) in mice | [92] |
| Antibacterial | In vitro | [51] |
| Antibacterial and antibiofilm effect: –inhibition of growth of P. aeruginosa; –depolarization of bacterial P. aeruginosa membrane; –inhibition of biofilm formation due to suppressed secretion of pyoverdine and suppressed secretion of protease and swarming motility of P. aeruginosa | In vivo Mouse model of catheter infection for evaluation of antibiofilm activity and BALB/c mouse model for determination of in vivo toxicity In vitro P. aeruginosa cultures; murine macrophage cell line (RAW 264.7) for cytotoxicity assay | [91] |
| Antibacterial against S. aureus Antifungal against C. albicans | In vitro | [28] |
| Antibacterial against S. aureus | In vitro | [81] |
| Bacteriostatic against S. aureus: –inhibition of extracellular protease production resulting in inhibition of S. aureus growth | In vitro | [66] |
| Antivirus: inhibition of virus HIV-1 protease | In vitro | [71] |
| Insect antifeedant | In vivo Spodoptera littoralis L6 larvae | [41] |
| Neuroprotective: –protecting against neurotoxicity (preventing neuronal loss); –blocking MPP+-induced apoptosis; –inhibiting intracellular accumulation of reactive oxygen species (ROS); –protecting from neuronal death through reversing the inhibition of the PI3-K/Akt/GSK3b pathway | In vitro Parkinson’s disease cellular model: MPP+-induced neurotoxicity in human neuroblastoma SH-SY5Y cells | [109] |
| Neuroprotective: –decreasing amyloid plaque deposition; –reducing microglial activation and decreasing the secretion of pro-inflammatory factors; –suppressing the production of pro-inflammatory markers and the nuclear translocation of nuclear factor-κB (NF-κB); –reducing inhibited neurotoxicity and improving neuron survival | In vivo Amyloid β precursor protein (APP)/presenilin 1 (PS1) transgenic mice In vitro BV2 microglia cells | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olech, M.; Ziemichód, W.; Nowacka-Jechalke, N. The Occurrence and Biological Activity of Tormentic Acid—A Review. Molecules 2021, 26, 3797. https://doi.org/10.3390/molecules26133797
Olech M, Ziemichód W, Nowacka-Jechalke N. The Occurrence and Biological Activity of Tormentic Acid—A Review. Molecules. 2021; 26(13):3797. https://doi.org/10.3390/molecules26133797
Chicago/Turabian StyleOlech, Marta, Wojciech Ziemichód, and Natalia Nowacka-Jechalke. 2021. "The Occurrence and Biological Activity of Tormentic Acid—A Review" Molecules 26, no. 13: 3797. https://doi.org/10.3390/molecules26133797
APA StyleOlech, M., Ziemichód, W., & Nowacka-Jechalke, N. (2021). The Occurrence and Biological Activity of Tormentic Acid—A Review. Molecules, 26(13), 3797. https://doi.org/10.3390/molecules26133797