Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds
Abstract
:1. Introduction
2. Challenges and Opportunities to Heal Exudative Wounds
2.1. Wounds Stages
2.1.1. Haemostasias
2.1.2. Inflammation
2.1.3. Proliferation/Maturation/Remodeling
2.2. Current Needs Regarding Exudative Wounds
2.3. Different Types of Exudate
2.4. Limitations of the Current Wound Dressing Approaches for Exudative Wounds
3. Aerogel Technology
3.1. Dressing Films
3.2. Bioaerogel Scaffolds
3.3. Bioaerogel Particles
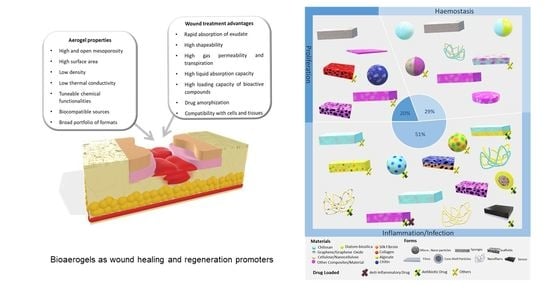
4. Bioaerogels Application Versus Wound Stages
4.1. Haemostasis
4.2. Inflammatory
4.3. Proliferative
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Enoch, S.; John Leaper, D. Basic Science of Wound Healing. Basic Sci. 2008, 26, 31–37. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-Based Biomaterials for Accelerated Diabetic Wound Healing: A Critical Review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for Wound Healing: Scope and Advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current Wound Healing Procedures and Potential Care. Mater. Sci. Eng. C 2015, 48, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the Chronic Wound: A Practical Approach to the Care of Nonhealing Wounds and Wound Care Dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef]
- Falanga, V. Wound Healing and Its Impairment in the Diabetic Foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Monaco, J.L.; Lawrence, W.T. Acute Wound Healing. Clin. Plast. Surg. 2003, 30, 1–12. [Google Scholar] [CrossRef]
- Rodero, M.P.; Khosrotehrani, K. Skin Wound Healing Modulation by Macrophages. Int. J. Clin. Exp. Pathol. 2010, 3, 643–653. [Google Scholar]
- Pinto, A.M.; Cerqueira, M.A.; Bañobre-Lópes, M.; Pastrana, L.M.; Sillankorva, S. Bacteriophages for Chronic Wound Treatment: From Traditional to Novel Delivery Systems. Viruses 2020, 12, 235. [Google Scholar] [CrossRef] [Green Version]
- Howell-Jones, R.S.; Wilson, M.J.; Hill, K.E.; Howard, A.J.; Price, P.E.; Thomas, D.W. A Review of the Microbiology, Antibiotic Usage and Resistance in Chronic Skin Wounds. J. Antimicrob. Chemother. 2005, 55, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.; Gómez, B.; McIntyre, M.; Dubick, M.; Christy, R.; Nicholson, S.; Burmeister, D. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef] [Green Version]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The Humanistic and Economic Burden of Chronic Wounds: A Protocol for a Systematic Review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- European Association for Injury Prevention and Safety Promotion (EuroSafe). EuroSafe: Injuries in the European Union. In Summary on Injury Statistics 2012–2014; Routledge: Amsterdam, The Netherland, 2016. [Google Scholar]
- Smith, F.; Dryburgh, N.; Donaldson, J.; Mitchell, M. Debridement for surgical wounds. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [Green Version]
- Banjare, J.; Bhalerao, S. Obesity Associated Noncommunicable Disease Burden. Int. J. Heal. Allied Sci. 2016, 5, 81. [Google Scholar] [CrossRef]
- Keast, D.H.; Bowering, C.K.; Evans, A.W.; Mackean, G.L.; Burrows, C.; D’Souza, L. MEASURE: A Proposed Assessment Framework for Developing Best Practice Recommendations for Wound Assessment. Wound Repair Regen. 2004, 12, s1–s17. [Google Scholar] [CrossRef]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.A.; Aquino, R.P.; Del Gaudio, P. Prilling and Supercritical Drying: A Successful Duo to Produce Core-Shell Polysaccharide Aerogel Beads for Wound Healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef]
- Soorbaghi, F.P.; Isanejad, M.; Salatin, S.; Ghorbani, M.; Jafari, S.; Derakhshankhah, H. Bioaerogels: Synthesis Approaches, Cellular Uptake, and the Biomedical Applications. Biomed. Pharmacother. 2019, 111, 964–975. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [Green Version]
- Yahya, E.B.; Amirul, A.A.; H.P.S., A.K.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; A.K., A.S.; Adnan, A.S. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- European Cooperation in Science and Technology (COST). COST CA18125—Advanced Engineering and Research of aeroGels for Environment and Life Sciences. Available online: https://cost-aerogels.eu/ (accessed on 5 January 2021).
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [Green Version]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Yahya, E.B.; Jummaat, F.; Amirul, A.A.; Adnan, A.S.; Olaiya, N.G.; Abdullah, C.K.; Rizal, S.; Mohamad Haafiz, M.K.; Khalil, H.P.S.A. A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef]
- Stergar, J.; Maver, U. Review of Aerogel-Based Materials in Biomedical Applications. J. Sol. Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, S.; Ying, Z.; Liu, J.; Zhou, Y.; Chen, F. Engineering of Aerogel-Based Biomaterials for Biomedical Applications. Int. J. Nanomedicine 2020, 15, 2363–2378. [Google Scholar] [CrossRef] [Green Version]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.F. Wound Exudate: Composition and Functions. Br. J. Community Nurs. 2003, 8 (Suppl. 3), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L. Exudate: Friend or Foe? Br. J. Community Nurs. 2014, 19, S18–S23. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, M.; Vowden, K.; Weir, D. Exudate Management Made Easy. Wounds Int. 2010, 1, 1–6. [Google Scholar]
- Merriam-Webster. “Exudate.” Merriam-Webster.com Dictionary. Available online: https://www.merriam-webster.com/dictionary/exudate (accessed on 2 November 2020).
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Maynard, J.; Collings, C. How Wounds Heal: The 4 Main Phases of Wound Healing. Available online: https://www.shieldhealthcare.com/ (accessed on 3 September 2020).
- Hasnain, M.S.; Nayak, A.K.; Alkahtani, S. (Eds.) Polymeric and Natural Composites, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Tsirogianni, A.K.; Moutsopoulos, N.M.; Moutsopoulos, H.M. Wound Healing: Immunological Aspects. Injury 2006, 37, S5–S12. [Google Scholar] [CrossRef]
- Nour, S.; Baheiraei, N.; Imani, R.; Khodaei, M.; Alizadeh, A.; Rabiee, N.; Moazzeni, S.M. A Review of Accelerated Wound Healing Approaches: Biomaterial-Assisted Tissue Remodeling. J. Mater. Sci. Mater. Med. 2019, 30, 120. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound Repair at a Glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [Green Version]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and Wound Repair: When Enough Is Enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]
- World Union of Wound Healing Societies (WUWHS) Consensus Document: Wound Exudate: Effective Assessment and Management; London, Wounds Int. 2019. Available online: https://www.woundsinternational.com/ (accessed on 3 September 2020).
- Wounds UK. Best Practice Statement: Effective Exudate Management. Wounds UK 2013. Available online: https://www.wounds-uk.com/resources/details/best-practice-statement-effective-exudate-management (accessed on 3 September 2020).
- Dowsett C (2012) Management of Wound Exudate. Independent Nurse. Available online: www.independentnurse.co.uk/clinical-article/management-of-wound-exudate/63637/ (accessed on 3 September 2020).
- Vowden, K.; Vowden, P. Understanding Exudate Management and the Role of Exudate in the Healing Process. Br. J. Community Nurs. 2003, 8, S4–S13. [Google Scholar] [CrossRef]
- International Wound Infection Institute (IWII). Wound Infection in Clinical Practice. Wounds Int. 2016. Available online: https://www.woundinfection-institute.com/ (accessed on 3 September 2020).
- Fromantin, I.; Seyer, D.; Watson, S.; Rollot, F.; Elard, J.; Escande, M.C.; De Rycke, Y.; Kriegel, I.; Larreta Garde, V. Bacterial Floras and Biofilms of Malignant Wounds Associated with Breast Cancers. J. Clin. Microbiol. 2013, 51, 3368–3373. [Google Scholar] [CrossRef] [Green Version]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M.; Stoodley, P.; Swanson, T.; Tachi, M.; Wolcott, R.D. Consensus Guidelines for the Identification and Treatment of Biofilms in Chronic Nonhealing Wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef]
- Wounds UK. Best Practice Statement: Making Day-to-Day Management of Biofilm Simple. Wounds UK 2017, 1–33. Available online: https://www.wounds-uk.com/resources/details/best-practice-statement-making-daytoday-management-biofilm-simple (accessed on 3 September 2020).
- Kragh, K.N.; Hutchison, J.B.; Melaugh, G.; Rodesney, C.; Roberts, A.E.L.; Irie, Y.; Jensen, P.Ø.; Diggle, S.P.; Allen, R.J.; Gordon, V.; et al. Role of Multicellular Aggregates in Biofilm Formation. MBio 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hurlow, J.; Couch, K.; Laforet, K.; Bolton, L.; Metcalf, D.; Bowler, P. Clinical Biofilms: A Challenging Frontier in Wound Care. Adv. Wound Care 2015, 4, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Tickle, J. Wound Exudate: A Survey of Current Understanding and Clinical Competency. Br. J. Nurs. 2016, 25, 102–109. [Google Scholar] [CrossRef]
- Cutting, K.F.; White, R.J. Maceration of the Skin and Wound Bed 1: Its Nature and Causes. J. Wound Care 2002, 11, 275–278. [Google Scholar] [CrossRef]
- Vowden, P.; Bond, E.; Meuleneire, F. Managing High Viscosity Exudate. Wounds UK 2015, 11, 56–60. [Google Scholar]
- Bates-Jensen, B.; Schultz, G.; Ovington, L. Management of Exudate, Biofilms, and Infection. In Wound Care; Wolters Kluwer: Philadelphia, PA, USA, 2012; pp. 457–476. [Google Scholar]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [Green Version]
- World Union of Wound Healing Societies (WUWHS). Wound Exudate and the Role of Dressings—A Consensus Document; MEP Ltd: London, UK, 2007. [Google Scholar]
- Dowsett, C. Moisture in Wound Healing: Exudate Management. Br. J. Community Nurs. 2011, 16, S6–S12. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Rosenbaum, A.J.; Banerjee, S.; Rezak, K.M.; Uhl, R.L. Advances in Wound Management. J. Am. Acad. Orthop. Surg. 2018, 26, 833–843. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of Terms Relating to the Structure and Processing of Sols, Gels, Networks, and Inorganic-Organic Hybrid Materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Durães, L.; Maleki, H.; Vareda, J.P.; Lamy-Mendes, A.; Portugal, A. Exploring the Versatile Surface Chemistry of Silica Aerogels for Multipurpose Application. MRS Adv. 2017, 2, 3511–3519. [Google Scholar] [CrossRef]
- Liebner, F.; Pircher, N.; Schimper, C.; Haimer, E.; Rosenau, T. Aerogels: Cellulose-Based. Encycl. Biomed. Polym. Polym. Biomater. 2015, 11, 37–75. [Google Scholar]
- Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of Aerogels, Cryogels and Xerogels of Alginate: Effect of Molecular Weight, Gelation Conditions and Drying Method on Particles’ Micromeritics. Molecules 2019, 24, 1049. [Google Scholar] [CrossRef] [Green Version]
- Duong, H.M.; Lim, Z.K.; Nguyen, T.X.; Gu, B.; Penefather, M.P.; Phan-Thien, N. Compressed Hybrid Cotton Aerogels for Stopping Liquid Leakage. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 537, 502–507. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Li, X.; Wu, Y.; Tang, K.; Liu, J.; Zheng, X.; Wan, G. Injectable Antibacterial Cellulose Nanofiber/Chitosan Aerogel with Rapid Shape Recovery for Noncompressible Hemorrhage. Int. J. Biol. Macromol. 2020, 154, 1185–1193. [Google Scholar] [CrossRef]
- Jack, A.A.; Nordli, H.R.; Powell, L.C.; Farnell, D.J.J.; Pukstad, B.; Rye, P.D.; Thomas, D.W.; Chinga-Carrasco, G.; Hill, K.E. Cellulose Nanofibril Formulations Incorporating a Low-Molecular-Weight Alginate Oligosaccharide Modify Bacterial Biofilm Development. Biomacromolecules 2019, 20, 2953–2961. [Google Scholar] [CrossRef]
- Raman, S.P.; Keil, C.; Dieringer, P.; Hübner, C.; Bueno, A.; Gurikov, P.; Nissen, J.; Holtkamp, M.; Karst, U.; Haase, H.; et al. Alginate Aerogels Carrying Calcium, Zinc and Silver Cations for Wound Care: Fabrication and Metal Detection. J. Supercrit. Fluids 2019, 153, 104545. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-Loaded Chitosan Aerogel Particles for Chronic Wound Applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Bernard, I.; Marin, M.A.; Ward, K.R.; McHugh, M.A. Superabsorbent Alginate Aerogels. J. Supercrit. Fluids 2013, 79, 202–208. [Google Scholar] [CrossRef]
- Darpentigny, C.; Nonglaton, G.; Bras, J.; Jean, B. Highly Absorbent Cellulose Nanofibrils Aerogels Prepared by Supercritical Drying. Carbohydr. Polym. 2020, 229, 115560. [Google Scholar] [CrossRef] [PubMed]
- Cerciello, A.; Del Gaudio, P.; Granata, V.; Sala, M.; Aquino, R.P.; Russo, P. Synergistic Effect of Divalent Cations in Improving Technological Properties of Cross-Linked Alginate Beads. Int. J. Biol. Macromol. 2017, 101, 100–106. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-Based Aerogels—Promising Biodegradable Carriers for Drug Delivery Systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Amorphization of Drugs by Adsorptive Precipitation from Supercritical Solutions: A Review. J. Supercrit. Fluids 2018, 132, 105–125. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, J.; Ding, J.; Van der Bruggen, B.; Shen, J.; Gao, C. Electric-Pulse Layer-by-Layer Assembled of Anion Exchange Membrane with Enhanced Monovalent Selectivity. J. Memb. Sci. 2018, 548, 81–90. [Google Scholar] [CrossRef]
- Lovskaya, D.; Menshutina, N.; Mochalova, M.; Nosov, A.; Grebenyuk, A. Chitosan-Based Aerogel Particles as Highly Effective Local Hemostatic Agents. Production Process and in Vivo Evaluations. Polymers 2020, 12, 2055. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Gurikov, P.; Monteiro, F.J.; Smirnova, I.; Alvarez-Lorenzo, C.; García-González, C.A. Jet Cutting Technique for the Production of Chitosan Aerogel Microparticles Loaded with Vancomycin. Polymers 2020, 12, 273. [Google Scholar] [CrossRef] [Green Version]
- Del Gaudio, P.; Auriemma, G.; Mencherini, T.; Porta, G.D.; Reverchon, E.; Aquino, R.P. Design of Alginate-Based Aerogel for Nonsteroidal Anti-Inflammatory Drugs Controlled Delivery Systems Using Prilling and Supercritical-Assisted Drying. J. Pharm. Sci. 2013, 102, 185–194. [Google Scholar] [CrossRef]
- Pantić, M.; Horvat, G.; Knez, Ž.; Novak, Z. Preparation and Characterization of Chitosan-Coated Pectin Aerogels: Curcumin Case Study. Molecules 2020, 25, 1187. [Google Scholar] [CrossRef] [Green Version]
- Batista, M.P.; Gonçalves, V.S.S.; Gaspar, F.B.; Nogueira, I.D.; Matias, A.A.; Gurikov, P. Novel Alginate-Chitosan Aerogel Fibres for Potential Wound Healing Applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Liu, C.; Yu, F.; Ding, X.; Li, T.; Hu, Q.; Liu, M.; Fang, F.; Xin, H.; Wang, X. Natural Extracted Aerogels with Inherent Anisotropy and Their 3D Printing Assisted Biomedical Applications. J. Mater. Chem. B 2017, 5, 6217–6220. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Marin, M.A.; Surampudi, V.; Subia, B.; Rao, R.R.; Kundu, S.C.; McHugh, M.A. Silk Fibroin Aerogels: Potential Scaffolds for Tissue Engineering Applications. Biomed. Mater. 2015, 10, 035002. [Google Scholar] [CrossRef]
- Ghafari, R.; Jonoobi, M.; Amirabad, L.M.; Oksman, K.; Taheri, A.R. Fabrication and Characterization of Novel Bilayer Scaffold from Nanocellulose Based Aerogel for Skin Tissue Engineering Applications. Int. J. Biol. Macromol. 2019, 136, 796–803. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An Emerging Platform for Drug Delivery: Aerogel Based Systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and Biomedical Applications of Aerogels: Possibilities and Challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Guenther, U.; Smirnova, I.; Neubert, R. Hydrophilic Silica Aerogels as Dermal Drug Delivery Systems–Dithranol as a Model Drug. Eur. J. Pharm. Biopharm. 2008, 69, 935–942. [Google Scholar] [CrossRef]
- Smirnova, I.; Mamic, J.; Arlt, W. Adsorption of Drugs on Silica Aerogels. Langmuir 2003, 19, 8521–8525. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan Preparations for Wounds and Burns: Antimicrobial and Wound-Healing Effects. Expert Rev. Anti. Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite Aerogels Based on Dialdehyde Nanocellulose and Collagen for Potential Applications as Wound Dressing and Tissue Engineering Scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Dharunya, G.; Duraipandy, N.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Curcumin Cross-Linked Collagen Aerogels with Controlled Anti-Proteolytic and pro-Angiogenic Efficacy. Biomed. Mater. 2016, 11, 045011. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C. P(TA) Macro-, Micro-, Nanoparticle-Embedded Super Porous p(HEMA) Cryogels as Wound Dressing Material. Mater. Sci. Eng. C 2017, 70, 317–326. [Google Scholar] [CrossRef]
- Tako, M. The Principle of Polysaccharide Gels. Adv. Biosci. Biotechnol. 2015, 6, 22–36. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and Biodegradable Hydrogels: Gelation, Biodegradation and Biomedical Applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-Based Composite Materials for Wound Dressing Application: A Mini Review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Mustapa, A.N.; Martin, A.; Sanz-Moral, L.M.; Rueda, M.; Cocero, M.J. Impregnation of Medicinal Plant Phytochemical Compounds into Silica and Alginate Aerogels. J. Supercrit. Fluids 2016, 116, 251–263. [Google Scholar] [CrossRef]
- Franco, P.; Pessolano, E.; Belvedere, R.; Petrella, A.; De Marco, I. Supercritical Impregnation of Mesoglycan into Calcium Alginate Aerogel for Wound Healing. J. Supercrit. Fluids 2020, 157, 104711. [Google Scholar] [CrossRef]
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of Antimicrobial and Scar Preventive Chitosan Hydrogel Wound Dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef]
- Ko, E.; Kim, H. Preparation of Chitosan Aerogel Crosslinked in Chemical and Ionical Ways by Non-Acid Condition for Wound Dressing. Int. J. Biol. Macromol. 2020, 164, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose Nanofiber Aerogel as a Promising Biomaterial for Customized Oral Drug Delivery. Int. J. Nanomedicine 2017, 12, 2021–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, C.; Jiao, Y.; Wei, S.; Zhang, L.; Wu, Y.; Li, J. Functional Nanocomposites from Sustainable Regenerated Cellulose Aerogels: A Review. Chem. Eng. J. 2019, 359, 459–475. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug Release from Nanoparticles Embedded in Four Different Nanofibrillar Cellulose Aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef]
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D.; et al. Preparation of Chitosan-Collagen-Alginate Composite Dressing and Its Promoting Effects on Wound Healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-Porosity Aerogels of High Specific Surface Area Prepared from Nanofibrillated Cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of Nanocellulose Scaffolds with Tunable Structures to Support 3D Cell Culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Li, V.C.F.; Mulyadi, A.; Dunn, C.K.; Deng, Y.; Qi, H.J. Direct Ink Write 3D Printed Cellulose Nanofiber Aerogel Structures with Highly Deformable, Shape Recoverable, and Functionalizable Properties. ACS Sustain. Chem. Eng. 2018, 6, 2011–2022. [Google Scholar] [CrossRef]
- Pircher, N.; Veigel, S.; Aigner, N.; Nedelec, J.M.; Rosenau, T.; Liebner, F. Reinforcement of Bacterial Cellulose Aerogels with Biocompatible Polymers. Carbohydr. Polym. 2014, 111, 505–513. [Google Scholar] [CrossRef]
- Della Porta, G.; Del Gaudio, P.; De Cicco, F.; Aquino, R.P.; Reverchon, E. Supercritical Drying of Alginate Beads for the Development of Aerogel Biomaterials: Optimization of Process Parameters and Exchange Solvents. Ind. Eng. Chem. Res. 2013, 52, 12003–12009. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, Z.; Zhao, Y.; Zou, Z.; Lu, S.; Zhang, B.; Li, S. Sponges of Carboxymethyl Chitosan Grafted with Collagen Peptides for Wound Healing. Int. J. Mol. Sci. 2019, 20, 3890. [Google Scholar] [CrossRef] [Green Version]
- Prüsse, U.; Bilancetti, L.; Bučko, M.; Bugarski, B.; Bukowski, J.; Gemeiner, P.; Lewińska, D.; Manojlovic, V.; Massart, B.; Nastruzzi, C.; et al. Comparison of Different Technologies for Alginate Beads Production. Chem. Pap. 2008, 62, 364. [Google Scholar] [CrossRef]
- Bian, H.; Shao, Z.; Li, L.; Liu, J.; Chen, K.; Zhang, X. Preparation of Diameter-Controlled Cellulose Aerogel Spheres via Atomization Method and Their Load Performance. Macromol. Mater. Eng. 2020, 305, 2000243. [Google Scholar] [CrossRef]
- Sescousse, R.; Gavillon, R.; Budtova, T. Wet and Dry Highly Porous Cellulose Beads from Cellulose–NaOH–Water Solutions: Influence of the Preparation Conditions on Beads Shape and Encapsulation of Inorganic Particles. J. Mater. Sci. 2011, 46, 759–765. [Google Scholar] [CrossRef]
- Omura, T.; Imagawa, K.; Kono, K.; Suzuki, T.; Minami, H. Encapsulation of Either Hydrophilic or Hydrophobic Substances in Spongy Cellulose Particles. ACS Appl. Mater. Interfaces 2017, 9, 944–949. [Google Scholar] [CrossRef]
- Druel, L.; Kenkel, A.; Baudron, V.; Buwalda, S.; Budtova, T. Cellulose Aerogel Microparticles via Emulsion-Coagulation Technique. Biomacromolecules 2020, 21, 1824–1831. [Google Scholar] [CrossRef]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel Microspheres from Natural Cellulose Nanofibrils and Their Application as Cell Culture Scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef]
- Quan, K.; Li, G.; Luan, D.; Yuan, Q.; Tao, L.; Wang, X. Black Hemostatic Sponge Based on Facile Prepared Cross-Linked Graphene. Colloids Surfaces B Biointerfaces 2015, 132, 27–33. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. A Review on Recent Advances in Chitosan Based Composite for Hemostatic Dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.; Li, G.; Tao, L.; Xie, Q.; Yuan, Q.; Wang, X. Diaminopropionic Acid Reinforced Graphene Sponge and Its Use for Hemostasis. ACS Appl. Mater. Interfaces 2016, 8, 7666–7673. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liang, Y.; Xu, C.; Sun, H.; Tao, L.; Wei, Y.; Wang, X. Polydopamine Reinforced Hemostasis of a Graphene Oxide Sponge via Enhanced Platelet Stimulation. Colloids Surfaces B Biointerfaces 2019, 174, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, X.; Zhang, K.; Yang, G.; Mu, Y.; Su, C.; Pang, J.; Chen, T.; Chen, X.; Feng, C. Chitosan/Diatom-Biosilica Aerogel with Controlled Porous Structure for Rapid Hemostasis. Adv. Healthc. Mater. 2020, 9, 2000951. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lv, L.; Li, Y.; Ren, X.; Luo, H.; Gao, Y.; Yan, H.; Li, Y.; Qu, Y.; Yang, L.; et al. Preparation and Evaluation of Bletilla Striata Polysaccharide/Graphene Oxide Composite Hemostatic Sponge. Int. J. Biol. Macromol. 2019, 130, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Mellado, C.; Figueroa, T.; Báez, R.; Castillo, R.; Melendrez, M.; Schulz, B.; Fernández, K. Development of Graphene Oxide Composite Aerogel with Proanthocyanidins with Hemostatic Properties As a Delivery System. ACS Appl. Mater. Interfaces 2018, 10, 7717–7729. [Google Scholar] [CrossRef]
- Figueroa, T.; Carmona, S.; Guajardo, S.; Borges, J.; Aguayo, C.; Fernández, K. Synthesis and Characterization of Graphene Oxide Chitosan Aerogels Reinforced with Flavan-3-Ols as Hemostatic Agents. Colloids Surfaces B Biointerfaces 2021, 197, 111398. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, J.; Wu, J.; Ding, S.; Yang, J.; Zhang, J.; Dong, A.; Deng, L. N-Alkylated Chitosan/Graphene Oxide Porous Sponge for Rapid and Effective Hemostasis in Emergency Situations. Carbohydr. Polym. 2019, 219, 405–413. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, C.; Liu, F.; Du, S.; Li, G.; Wang, X. Eliminating Heat Injury of Zeolite in Hemostasis via Thermal Conductivity of Graphene Sponge. ACS Appl. Mater. Interfaces 2019, 11, 23848–23857. [Google Scholar] [CrossRef]
- Aramwit, P.; Ratanavaraporn, J.; Siritientong, T. Improvement of Physical and Wound Adhesion Properties of Silk Sericin and Polyvinyl Alcohol Dressing Using Glycerin. Adv. Skin Wound Care 2015, 28, 358–367. [Google Scholar] [CrossRef]
- Pandit, A.P.; Koyate, K.R.; Kedar, A.S.; Mute, V.M. Spongy Wound Dressing of Pectin/Carboxymethyl Tamarind Seed Polysaccharide Loaded with Moxifloxacin Beads for Effective Wound Heal. Int. J. Biol. Macromol. 2019, 140, 1106–1115. [Google Scholar] [CrossRef]
- Keil, C.; Hübner, C.; Richter, C.; Lier, S.; Barthel, L.; Meyer, V.; Subrahmanyam, R.; Gurikov, P.; Smirnova, I.; Haase, H. Ca-Zn-Ag Alginate Aerogels for Wound Healing Applications: Swelling Behavior in Simulated Human Body Fluids and Effect on Macrophages. Polymers 2020, 12, 2741. [Google Scholar] [CrossRef]
- Edwards, J.; Fontenot, K.; Liebner, F.; Condon, B. Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity. Sensors 2018, 18, 2334. [Google Scholar] [CrossRef] [Green Version]
- Mehrabani, M.G.; Karimian, R.; Mehramouz, B.; Rahimi, M.; Kafil, H.S. Preparation of Biocompatible and Biodegradable Silk Fibroin/Chitin/Silver Nanoparticles 3D Scaffolds as a Bandage for Antimicrobial Wound Dressing. Int. J. Biol. Macromol. 2018, 114, 961–971. [Google Scholar] [CrossRef]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/Silk Fibroin/TiO2 Bio-Nanocomposite as a Biocompatible Wound Dressing Bandage with Strong Antimicrobial Activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Piątkowski, M.; Janus, Ł.; Bogdał, D.; Matysek, D.; Cablik, V. Microwave-Assisted Synthesis and Characterization of Antioxidant Chitosan-Based Aerogels for Biomedical Applications. Int. J. Polym. Anal. Charact. 2018, 23, 721–729. [Google Scholar] [CrossRef]
- Valchuk, N.A.; Brovko, O.S.; Palamarchuk, I.A.; Boitsova, T.A.; Bogolitsyn, K.G.; Ivakhnov, A.D.; Chukhchin, D.G.; Bogdanovich, N.I. Preparation of Aerogel Materials Based on Alginate–Chitosan Interpolymer Complex Using Supercritical Fluids. Russ. J. Phys. Chem. B 2019, 13, 1121–1124. [Google Scholar] [CrossRef]
- Jack, A.A.; Nordli, H.R.; Powell, L.C.; Powell, K.A.; Kishnani, H.; Johnsen, P.O.; Pukstad, B.; Thomas, D.W.; Chinga-Carrasco, G.; Hill, K.E. The Interaction of Wood Nanocellulose Dressings and the Wound Pathogen P. Aeruginosa. Carbohydr. Polym. 2017, 157, 1955–1962. [Google Scholar] [CrossRef]
- Darpentigny, C.; Marcoux, P.R.; Menneteau, M.; Michel, B.; Ricoul, F.; Jean, B.; Bras, J.; Nonglaton, G. Antimicrobial Cellulose Nanofibril Porous Materials Obtained by Supercritical Impregnation of Thymol. ACS Appl. Bio Mater. 2020, 3, 2965–2975. [Google Scholar] [CrossRef]
- Edwards, J.; Fontenot, K.; Prevost, N.; Pircher, N.; Liebner, F.; Condon, B. Preparation, Characterization and Activity of a Peptide-Cellulosic Aerogel Protease Sensor from Cotton. Sensors 2016, 16, 1789. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.V.; Fontenot, K.; Liebner, F.; Nee Pircher, N.D.; French, A.D.; Condon, B.D. Structure/Function Analysis of Cotton-Based Peptide-Cellulose Conjugates: Spatiotemporal/Kinetic Assessment of Protease Aerogels Compared to Nanocrystalline and Paper Cellulose. Int. J. Mol. Sci. 2018, 19, 840. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, D.; Duraipandy, N.; Srivatsan, K.V.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Fabrication of Hybrid Collagen Aerogels Reinforced with Wheat Grass Bioactives as Instructive Scaffolds for Collagen Turnover and Angiogenesis for Wound Healing Applications. ACS Appl. Mater. Interfaces 2017, 9, 16939–16950. [Google Scholar] [CrossRef]
- Concha, M.; Vidal, A.; Giacaman, A.; Ojeda, J.; Pavicic, F.; Oyarzun-Ampuero, F.A.; Torres, C.; Cabrera, M.; Moreno-Villoslada, I.; Orellana, S.L. Aerogels Made of Chitosan and Chondroitin Sulfate at High Degree of Neutralization: Biological Properties toward Wound Healing. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2018, 106, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Shukla, S.; Khan, I.; Kang, S.-M.; Haldorai, Y.; Tripathi, K.M.; Jung, S.; Chen, L.; Kim, T.; Huh, Y.S.; et al. A Sustainable Graphene Aerogel Capable of the Adsorptive Elimination of Biogenic Amines and Bacteria from Soy Sauce and Highly Efficient Cell Proliferation. ACS Appl. Mater. Interfaces 2019, 11, 43949–43963. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. In Vitro and in Vivo Evaluation of a Novel Collagen/Cellulose Nanocrystals Scaffold for Achieving the Sustained Release of Basic Fibroblast Growth Factor. J. Biomater. Appl. 2015, 29, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of Copper-Containing Mesoporous Bioactive Glass and Nanofibrillated Cellulose: Biocompatibility and Angiogenic Promotion in Chronic Wound Healing Application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef]
- Orellana, S.L.; Giacaman, A.; Vidal, A.; Morales, C.; Oyarzun-Ampuero, F.; Lisoni, J.G.; Henríquez-Báez, C.; Morán-Trujillo, L.; Concha, M.; Moreno-Villoslada, I. Chitosan/Chondroitin Sulfate Aerogels with High Polymeric Electroneutralization Degree: Formation and Mechanical Properties. Pure Appl. Chem. 2018, 90, 901–911. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Zhao, Y.; Gao, H.; Shi, X.; Cai, J.; Deng, H.; Chen, Y.; Du, Y. Electroassembly of Chitin Nanoparticles to Construct Freestanding Hydrogels and High Porous Aerogels for Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 34766–34776. [Google Scholar] [CrossRef]
- Weng, L.; Boda, S.K.; Wang, H.; Teusink, M.J.; Shuler, F.D.; Xie, J. Novel 3D Hybrid Nanofiber Aerogels Coupled with BMP-2 Peptides for Cranial Bone Regeneration. Adv. Healthc. Mater. 2018, 7, 1701415. [Google Scholar] [CrossRef]
- Santo, V.E.; Duarte, A.R.C.; Popa, E.G.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Enhancement of osteogenic differentiation of human adipose derived stem cells by the controlled release of platelet lysates from hybrid scaffolds produced by supercritical fluid foaming. J. Control. Release. 2012, 162, 19–27. [Google Scholar] [CrossRef]
- Ribeiro, N.; Soares, G.C.; Santos-Rosales, V.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A.; Oliveira, A.L. A new era for sterilization based on supercritical CO2 technology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 399–428. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, A.; García-González, C. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach. Molecules 2019, 24, 871. [Google Scholar] [CrossRef] [Green Version]
- Soares, G.C.; Learmonth, D.A.; Vallejo, M.C.; Davila, S.P.; González, P.; Sousa, R.A.; Oliveira, A.L. Supercritical CO2 technology: The next standard sterilization technique? Mater. Sci. Eng. 2019, 99, 520–540. [Google Scholar] [CrossRef]
- Duarte, M.M.; Ribeiro, N.; Silva, I.V.; Dias, J.R.; Alves, N.M.; Oliveira, A.L. Fast decellularization process using supercritical carbon dioxide for trabecular bone. J. Supercrit. Fluids 2021, 172, 105194. [Google Scholar] [CrossRef]







| Examples of Factors | High Levels Exudation | Low Levels Exudation |
|---|---|---|
| Types of wounds | Chronic Venous Leg Ulcers Dehisced Surgical Wounds Malignant fungating wounds Burns Inflammatory ulcers—e.g., rheumatoid ulcers, pyoderma gangrenosum Skin donor sites | Ischemic/arterial wounds Neuropathic diabetic foot ulcers |
| Wound healing phase | Acute wounds—Beginning of healing process—inflammatory phase Chronic Wounds—prolonged inflammatory response | Towards end of healing process—proliferation and maturation phases |
| Wound size and depth | larger and deeper wounds | Smaller and superficial |
| Type | Pictures | Color/Opacity | Consistency | Interpretations/observations |
|---|---|---|---|---|
| Serous |  | Clear, amber or straw-colored | Thin, watery | Normal during inflammatory and proliferative phases of healing Increase in in this type exudate may be a sign of infection or decompensation of comorbidities (e.g. Cardiac failure or malnutrition) |
| Serosanguineous |  | Clear, pink to light red | Thin, slightly thicker than water | May be considered normal during inflammatory and proliferative phases of healing. Pinkish (Rosy) due to the presence of red blood cells. |
| Sanguineous |  | Red | Thin, watery | Reddish (Rose-colored) due to the presence of red blood cells. May indicate new blood vessel growth or the disruption of blood vessels or Capillary damage. Hypergranulation may be present. |
| Seropurulent |  | Cloudy, creamy, yellow, or tan | Thin | Serous exudate containing pus (Presence of inflammatory cells, neutrophils and microorganisms). May also be due to liquefying necrotic tissue. May signal impending infection. |
| Fibrinous |  | Cloudy | Thin, watery | May be associated to presence of fibrin strands. May indicate inflammation, with or without infection. |
| Purulent |  | Opaque, milky, yellow, tan or brown, sometimes green | Viscous, sticky | Essentially pus Indicates infection. Specific coloration may be due to particular infection (e.g., Green coloration- Pseudomonas aeruginosa). |
| Haemopurulent |  | Reddish, milky, opaque | Viscous | Mixture of blood and pus. Often due to established infection. |
| Hemorrhagic |  | Dark red | Viscous | May be associated with Trauma. Capillary Friability. May indicate bacterial infection. |
| Status | Pictures | Indicators | Comments |
|---|---|---|---|
| Dry |  | Wound bed is dry; there is no visible moisture and the primary dressing is unmarked; Not an ideal wound healing environment; | Dressing may be adherent to wound. Dressing material with no signs of exudation Dry perilesional skin |
| Moist |  | Small amounts of fluid are visible when the dressing is removed; wound bed could appear glossy; | Good exudate management—dressing change frequency is appropriate for dressing type; Conditions surrounding skin may be intact and hydrated. |
| Wet |  | Small amounts of fluid are visible when the dressing is removed; the primary dressing may have absorbed large amounts of exudation | Apparent imbalance in exudate management—Dressing material passed through but not saturated. Dressing material has absorbed large amounts of exudate, assess frequency of dressing change which may be appropriate Potential for peri wound maceration |
| Saturated |  | Primary dressing is wet and may be saturated; Not an ideal wound healing environment; | Imbalance in exudate management. It is necessary to simultaneously adjust the frequency of dressing change (dressing change is required more frequently than usual for the dressing type). Maceration and loss of perilesional skin integrity may be present. |
| Leaking |  | Dressings are saturated and exudate is escaping from primary and secondary dressings onto clothes or beyond; Not an ideal wound healing environment; | Mismanagement of exudate. Possibility of material degradation. Dressing change is required much more frequently than usual for dressing type. Macerated surrounding skin with loss of skin integrity |
| Clinical Need and Indications for Use |
|---|
|
|
|
|
|
|
|
| Allergies/sensitivities to dressing materials or contents |
| Availability/inclusion in local formulary |
| Reimbursement policies |
| Dressing/device change frequency Clinician preference/experience Economic capacity of the Patient and particular preferences |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardes, B.G.; Del Gaudio, P.; Alves, P.; Costa, R.; García-Gonzaléz, C.A.; Oliveira, A.L. Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds. Molecules 2021, 26, 3834. https://doi.org/10.3390/molecules26133834
Bernardes BG, Del Gaudio P, Alves P, Costa R, García-Gonzaléz CA, Oliveira AL. Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds. Molecules. 2021; 26(13):3834. https://doi.org/10.3390/molecules26133834
Chicago/Turabian StyleBernardes, Beatriz G., Pasquale Del Gaudio, Paulo Alves, Raquel Costa, Carlos A. García-Gonzaléz, and Ana Leite Oliveira. 2021. "Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds" Molecules 26, no. 13: 3834. https://doi.org/10.3390/molecules26133834
APA StyleBernardes, B. G., Del Gaudio, P., Alves, P., Costa, R., García-Gonzaléz, C. A., & Oliveira, A. L. (2021). Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds. Molecules, 26(13), 3834. https://doi.org/10.3390/molecules26133834