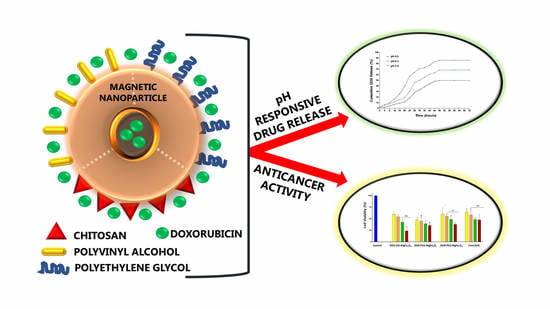
Chitosan, Polyethylene Glycol and Polyvinyl Alcohol Modified MgFe2O4 Ferrite Magnetic Nanoparticles in Doxorubicin Delivery: A Comparative Study In Vitro
Abstract
:1. Introduction
2. Results
2.1. Characterization of MNPs
2.2. Encapsulation Efficiency
2.3. TEM and NTA Studies
2.4. DOX Release
2.5. MTT Cytotoxicity
2.6. Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of MgFe2O4 Magnetic Nanoparticles (MNPs)
4.3. Functionalization of the MgFe2O4 MNPs with Chitosan (CHI)
4.4. Functionalization of the MgFe2O4 MNPs with Polyvinyl Alcohol (PVA)
4.5. Functionalization of the MgFe2O4 MNPs with Polyethylene Glycol (PEG)
4.6. Fourier-Transform Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD), and Vibrating Sample Magnetometry (VSM)
4.7. Encapsulation of Doxorubicin (DOX)
4.8. Transmission Electron Microscopy (TEM), Nanoparticle Tracking Analysis (NTA)
4.9. In Vitro Drug Release
4.10. MTT Cytotoxicity Assay
4.11. Fluorescent Apoptosis Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic drug delivery: Where the field is going? Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009, 71, 431–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, E.A.; Suman, V.J.; Davidson, N.E.; Sledge, G.W.; Kaufman, P.A.; Hudis, C.A.; Martino, S.; Gralow, J.R.; Dakhil, S.R.; Ingle, J.N.; et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J. Clin. Oncol. 2008, 26, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, M.; Shamspur, T.; Fathirad, F. In situ Preparation of Magnetic Fe3O4 Nanoparticles in Presence of PLGA and PVA as Magnetite Nanocarrier for Targeted Drug Delivery. J. Pharm. Drug Deliv. Res. 2017, 6, 2. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440. [Google Scholar] [CrossRef]
- Qi, J.; Yao, P.; He, F.; Yu, C.; Huang, C. Nanoparticles with dextran/chitosan shell and BSA/chitosan core—Doxorubicin loading and delivery. Int. J. Pharm. 2010, 393, 177–185. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Z.; Shi, X. Dendrimer-based molecular imaging contrast agents. Prog. Polym. Sci. 2015, 44, 1–27. [Google Scholar] [CrossRef]
- Almaki, J.H.; Nasiri, R.; Idris, A.; Majid, F.A.A.; Salouti, M.; Wong, T.S.; Dabagh, S.; Marvibaigi, M.; Amini, N. Synthesis, characterization and in vitro evaluation of exquisite targeting SPIONs–PEG–HER in HER2+ human breast cancer cells. Nanotechnology 2016, 27, 105601. [Google Scholar] [CrossRef]
- Mngadi, S.; Mokhosi, S.; Singh, M.; Mdlalose, W.B. Chitosan-functionalized Mg0.5Co0.5Fe2O4 magnetic nanoparticles enhance delivery of 5-fluorouracil in vitro. Coatings 2020, 10, 446. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. A 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lee, J.; Kang, J.; Chung, C.H.; Lee, K.; Suh, J.S.; Yoon, H.G.; Huh, Y.M.; Haam, S. Magnetic sensitivity enhanced novel fluorescent magnetic silica nanoparticles for biomedical applications. Nanotechnology 2008, 19, 075610. [Google Scholar] [CrossRef] [PubMed]
- Behnam, B.; Rezazadehkermani, M.; Ahmadzadeh, S.; Mokhtarzadeh, A.; Nematollahi-Mahani, S.N.; Pardakhty, A. Microniosomes for concurrent doxorubicin and iron oxide nanoparticles loading; preparation, characterization and cytotoxicity studies. Artif. Cell Nanomed. B 2018, 46, 118–125. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm 2015, 496, 191–218. [Google Scholar] [CrossRef]
- Sadighian, S.; Rostamizadeh, K.; Hosseini-Monfared, H.; Hamidi, M. Doxorubicin-conjugated core–shell magnetite nanoparticles as dual-targeting carriers for anticancer drug delivery. Colloids Surf. B Biointerfaces 2014, 117, 406–413. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Esmaeili, A.; Hadad, N.A. Preparation of ZnFe2O4–chitosan-doxorubicin hydrochloride nanoparticles and investigation of their hyperthermic heat-generating characteristics. Ceram. Int. 2015, 41, 7529–7535. [Google Scholar] [CrossRef]
- Mngadi, S.M.; Mokhosi, S.R.; Singh, M. Surface-coating of Mg0.5Co0.5Fe2O4 nanoferrites and their in vitro cytotoxicity. Inorg. Chem. Commun. 2019, 108, 107525. [Google Scholar] [CrossRef]
- Padayachee, J.; Daniels, A.; Balgobind, A.; Ariatti, M.; Singh, M. HER2/neu and MYC gene silencing in breast cancer: Therapeutic potential and advancement in nonviral nanocarrier systems. Nanomedicine 2020, 15, 1437–1452. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurchania, R.; Sawant, S.S.; Ball, R.J. Synthesis and characterization of magnetite/polyvinyl alcohol core–shell composite nanoparticles. J. Amer. Ceram. Soc. 2014, 97, 3208–3215. [Google Scholar] [CrossRef]
- Lassenberger, A.; Scheberl, A.; Stadlbauer, A.; Stiglbauer, A.; Helbich, T.; Reimhult, E. Individually stabilized, superparamagnetic nanoparticles with controlled shell and size leading to exceptional stealth properties and high relativities. ACS Appl. Mater. Interfaces 2017, 9, 3343–3353. [Google Scholar] [CrossRef] [Green Version]
- Xiong, F.; Huang, S.; Gu, N. Magnetic nanoparticles: Recent developments in drug delivery system. Drug Dev. Ind. Pharm. 2018, 44, 697–706. [Google Scholar] [CrossRef]
- Kanagesan, S.; Hashim, M.; Tamilselvan, S.; Alitheen, N.B.; Ismail, I.; Bahmanrokh, G. Cytotoxic effect of nanocrystalline MgFe2O4 particles for cancer cure. J. Nanomater. 2013, 2013, 165. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, M.; Ahmad, M.; Akhtar, M.S.; Shaari, A.; Riaz, S.; Naseem, S.; Masood, M.; Saeed, M.A. Magnetic properties of polyvinyl alcohol and doxorubicin loaded iron oxide nanoparticles for anticancer drug delivery applications. PLoS ONE 2016, 11, e0158084. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Tang, S.; Tong, Q. Oleanolic acid liposomes with polyethylene glycol modification: Promising antitumor drug delivery. Int. J. Nanomed. 2012, 7, 3517. [Google Scholar] [CrossRef] [Green Version]
- Javid, A.; Ahmadian, S.; Saboury, A.A.; Kalantar, S.M.; Rezaei-Zarchi, S. Chitosan-coated superparamagnetic iron oxide nanoparticles for doxorubicin delivery: Synthesis and anticancer effect against human ovarian cancer cells. Chem. Biol. Drug Des. 2013, 82, 296–306. [Google Scholar] [CrossRef]
- Pham, X.N.; Nguyen, T.P.; Pham, T.N.; Tran, T.T.N.; Tran, T.V.T. Synthesis and characterization of chitosan-coated magnetite nanoparticles and their application in curcumin drug delivery. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045010. [Google Scholar] [CrossRef]
- Zhang, S.; Lü, T.; Qi, D.; Cao, Z.; Zhang, D.; Zhao, H. Synthesis of quaternized chitosan-coated magnetic nanoparticles for oil-water separation. Mater. Lett. 2017, 191, 128–131. [Google Scholar] [CrossRef]
- Ehi-Eromosele, C.O. The Effect of Polyethylene Glycol (PEG) Coating on the Magneto-Structural Properties and Colloidal Stability of Co0.8Mg0.2Fe2O4 Nanoparticles for Potential Biomedical Applications. Dig. J. Nanomater. Bios. 2016, 11, 7–14. [Google Scholar]
- Nayek, C.; Manna, K.; Bhattacharjee, G.; Murugavel, P.; Obaidat, I. Investigating size-and temperature-dependent coercivity and saturation magnetization in PEG coated Fe3O4 nanoparticles. Magnetochemistry 2017, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Venkateswarlu, K.; Sandhyarani, M.; Nellaippan, T.A.; Rameshbabu, N. Estimation of crystallite size, lattice strain and dislocation density of nanocrystalline carbonate substituted hydroxyapatite by X-ray peak variance analysis. Procedia Mater. Sci. 2014, 5, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Omer, M.I.; Elbadawi, A.A.; Yassin, O.A. Synthesis and structural properties of MgFe2O4 ferrite nano-particles. J. Appl. Indust. Sci. 2013, 1, 20–23. [Google Scholar]
- Maleki, H.; Simchi, A.; Imani, M.; Costa, B.F.O. Size-controlled synthesis of superparamagnetic iron oxide nanoparticles and their surface coating by gold for biomedical applications. J. Magn. Magn. Mater. 2012, 324, 3997–4005. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.; Haron, M.; Namvar, F.; Nadi, B.; Rahman, M.; Amin, J. Synthesis, surface modification and characterization of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huong, N.T.; Giang, L.T.K.; Binh, N.T. Surface modification of iron oxide nanoparticles and their conjunction with water soluble polymers for biomedical application. J. Phys. Conf. Ser. 2009, 187, 012046. [Google Scholar] [CrossRef]
- Köferstein, R.; Walther, T.; Hesse, D.; Ebbinghaus, S.G. Preparation and characterization of nanosized magnesium ferrite powders by a starch-gel process and corresponding ceramics. J. Mater. Sci. 2013, 48, 6509–6518. [Google Scholar] [CrossRef] [Green Version]
- Khot, V.M.; Salunkhe, A.B.; Thorat, N.D.; Ningthoujam, R.S.; Pawar, S.H. Induction heating studies of dextran coated MgFe2O4 nanoparticles for magnetic hyperthermia. Dalton Trans. 2013, 42, 1249–1258. [Google Scholar] [CrossRef]
- Agrawal, Y.K.; Patel, V.R. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81. [Google Scholar] [CrossRef]
- Oladimeji, O.; Akinyelu, J.; Singh, M. Co-Polymer Functionalised Gold Nanoparticles Show Efficient Mitochondrial Targeted Drug Delivery in Cervical Carcinoma Cells. J. Biomed. Nanotechnol. 2020, 16, 853–866. [Google Scholar] [CrossRef]
- Kievit, F.M.; Zhang, M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc. Chem. Res. 2011, 44, 853–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, U.; Adireddy, S.; Jaiswal, A.; Mandava, S.; Lee, B.; Chrisey, D. In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. Int. J. Mol. Sci. 2015, 16, 24417. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Q.; Chen, M.; Huang, Q.; Wang, W.; Li, Q.; Huang, Y.; Di, W. The Changing 50% Inhibitory Concentration (ic50) of Cisplatin: A Pilot Study on the Artifacts of the Mtt Assay and the Precise Measurement of Density-Dependent Chemoresistance in Ovarian Cancer. Oncotarget 2016, 7, 70803–70821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, Z.; Karimi, L.; Shokrollahi, H. Nano-magnetic particles used in biomedicine: Core and coating materials. Mater. Sci. Eng. C 2013, 33, 2465–2475. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Singh, A.; Mohanty, C.; Sahoo, S.K. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials 2010, 31, 3694–3706. [Google Scholar] [CrossRef]
- Akinyelu, J.; Singh, M. Folate-tagged chitosan functionalized gold nanoparticles for enhanced delivery of 5-fluorouracil to cancer cells. Appl. Nanosci. 2019, 9, 7–17. [Google Scholar] [CrossRef]
- Oladimeji, O.; Akinyelu, A.; Daniels, A.; Singh, M. Modified Gold Nanoparticles for efficient Delivery of Betulinic Acid to Cancer Cell Mitochondria. Int. J. Mol. Sci. 2021, 22, 5072. [Google Scholar] [CrossRef] [PubMed]
- Cirtiu, C.M.; Raychoudhury, T.; Ghoshal, S.; Moores, A. Systematic comparison of the size, surface characteristics and colloidal stability of zero valent iron nanoparticles pre-and post-grafted with common polymers. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 95–104. [Google Scholar] [CrossRef]
- Tang, H.B.; Li, L.; Chen, H.; Zhou, Z.M.; Chen, H.L.; Li, X.M.; Liu, L.R.; Wang, Y.S.; Zhang, Q.Q. Stability and in vivo evaluation of pullulan acetate as a drug nanocarrier. Drug Deliv. 2010, 17, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; He, Q. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behaviour. Expert Opin. Drug Deliv. 2014, 11, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, O.; Akinyelu, J.; Singh, M. Nanomedicines for Subcellular Targeting: The Mitochondrial Perspective. Curr. Med. Chem. 2020, 27, 5480–5509. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Salas, G.; Veintemillas-Verdaguer, S.; Morales, M.D.P. Relationship between physico-chemical properties of magnetic fluids and their heating capacity. Int. J. Hyperth. 2013, 29, 768–776. [Google Scholar] [CrossRef] [Green Version]
- Billotey, C.; Wilhelm, C.; Devaud, M.; Bacri, J.C.; Bittoun, J.; Gazeau, F. Cell internalization of anionic maghemite nanoparticles: Quantitative effect on magnetic resonance imaging. Magn. Reason Med. 2003, 49, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, P.; Lavergne, E.; Gazeau, F.; Lewin, M.; Boissonnas, A.; Doan, B.T.; Gillet, B.; Combadiere, C.; Combadiere, B.; Clément, O. In vivo cellular imaging of lymphocyte trafficking by MRI: A tumor model approach to cell-based anticancer therapy. Magn. Reson. Med. 2006, 56, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Shetty, A.B.; Hatami, E.; Chowdhury, P.; Yallapu, M.M. Pectin-Tannic Acid Nano-Complexes Promote the Delivery and Bioactivity of Drugs in Pancreatic Cancer Cells. Pharmaceutics 2020, 12, 285. [Google Scholar] [CrossRef] [Green Version]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eugenol-Containing Essential Oils Loaded onto Chitosan/Polyvinyl Alcohol Blended Films and Their Ability to Eradicate Staphylococcus aureus or Pseudomonas aeruginosa from infected Microenvironments. Pharmaceutics 2021, 13, 195. [Google Scholar] [CrossRef]
- Maney, V.; Singh, M. An in vitro assessment of Chitosan/ Bimetallic PtAu nanocomposites as delivery vehicles for Doxorubicin. Nanomedicine 2017, 12, 2625–2640. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Sterically Stabilized Polymeric Mesoporous Silica Nanoparticles Improve Doxorubicin Efficiency: Tailored Cancer Therapy. Molecules 2020, 25, 742. [Google Scholar] [CrossRef] [Green Version]
- Gounden, S.; Daniels, A.; Singh, M. Chitosan-Modified Silver Nanoparticles Enhance Cisplatin Activity in Breast Cancer Cells. Biointerface Res. Appl. Chem. 2021, 11, 10572–10584. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, D.; Li, C.; Jia, L.; Liu, G.; Hao, L.; Zheng, D.; Shen, J.; Li, T.; Guo, Y.; et al. Self-assembled nanoparticles based on galactosylated O-carboxymethyl chitosan-graft-stearic acid conjugates for delivery of doxorubicin. Int. J. Pharm. 2013, 458, 31–38. [Google Scholar] [CrossRef]
- Montha, W.; Maneeprakorn, W.; Buatong, N.; Tang, I.M.; Pon-On, W. Synthesis of doxorubicin-PLGA loaded chitosan stabilized (Mn, Zn) Fe2O4 nanoparticles: Biological activity and pH-responsive drug release. Mater. Sci. Eng. C 2016, 59, 235–240. [Google Scholar] [CrossRef]
- Voinov, M.A.; Pagán, J.O.S.; Morrison, E.; Smirnova, T.I.; Smirnov, A.I. Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J. Amer. Chem. Soc. 2010, 133, 35–41. [Google Scholar] [CrossRef]
- Frey, T. Nucleic acid dyes for detection of apoptosis in live cells. Cytom. A 1995, 21, 265–274. [Google Scholar] [CrossRef]
- Atale, N.; Gupta, S.; Yadav, U.C.S.; Rani, V. Cell-death assessment by fluorescent and non-fluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Liu, Y.P.; Ho, J.H.; Hsu, S.C.; Lee, O.K. Amine-surface-modified superparamagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J. Orthop. Res. 2012, 30, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Cai, H.; Wang, X.; Cao, X.; Li, K.; Wang, S.H.; Guo, R.; Zheng, L.; Zhang, G.; Shi, X. Facile one-pot preparation, surface functionalization, and toxicity assay of APTS-coated iron oxide nanoparticles. Nanotechnology 2012, 23, 105601. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef]
- Hoskins, C.; Cuschieri, A.; Wang, L. The cytotoxicity of polycationic iron oxide nanoparticles: Common endpoint assays and alternative approaches for improved understanding of cellular response mechanism. J. Nanobiotechnol. 2012, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarus, G.; Singh, M. Cationic modified gold nanoparticles show enhanced gene delivery in vitro. Nanotechnol. Rev. 2016, 5, 425–434. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Vishnubhotla, R.; Shan, S.; Chauhan, C.; Cho, M.; Glover, S.C. Higher Molecular Weight Polyethylene Glycol Increases Cell Proliferation While Improving Barrier Function in an In Vitro Colon Cancer Model. J. Biomed. Biotechnol. 2011, 2011, 587470. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Hu, K.-H.; Wei, Z.-H. Comparison of cell behavior on pva/ pva-gelatin electrospun nanofibers with random and aligned configuration. Sci. Rep. 2016, 6, 37960. [Google Scholar] [CrossRef]
- Box, V.G. The intercalation of DNA double helices with doxorubicin and nagalomycin. J. Mol. Graph. Model. 2007, 26, 14–19. [Google Scholar] [CrossRef]
- Soares, P.I.; Sousa, A.I.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Towards the development of multifunctional chitosan-based iron oxide nanoparticles: Optimization and modelling of doxorubicin release. Carbohydr. Polym. 2016, 153, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Dönmez, Y.; Gündüz, U. Reversal of multidrug resistance by small interfering RNA (siRNA) in doxorubicin-resistant MCF-7 breast cancer cells. Biomed. Pharmacother. 2011, 65, 85–89. [Google Scholar] [CrossRef]
- Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gunduz, U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur. J. Pharm. Sci. 2014, 62, 243–250. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Kopecka, J.; Rankin, G.M.; Salaroglio, I.C.; Poulsen, S.A.; Riganti, C. P-glycoprotein-mediated chemoresistance is reversed by carbonic anhydrase XII inhibitors. Oncotarget 2016, 7, 85861–85875. [Google Scholar] [CrossRef] [Green Version]
- Rose, P.A.; Praseetha, P.K.; Bhagat, M.; Alexander, P.; Abdeen, S.; Chavali, M. Drug embedded PVP coated magnetic nanoparticles for targeted killing of breast cancer cells. Technol. Cancer Res. Treat. 2013, 12, 463–472. [Google Scholar] [CrossRef]
- Hua, M.Y.; Yang, H.W.; Chuang, C.K.; Tsai, R.Y.; Chen, W.J.; Chuang, K.L.; Chang, Y.H.; Chuang, H.C.; Pang, S.T. Magnetic-nanoparticle-modified paclitaxel for targeted therapy for prostate cancer. Biomaterials 2010, 31, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, W.B.; Msomi, J.Z.; Moyo, T. XRD, Mössbauer and magnetic properties of MgxCo1−xFe2O4 nanoferrites. J. Magn. Magn. Mater. 2015, 373, 78–82. [Google Scholar] [CrossRef]
- Khalkhali, M.; Rostamizadeh, K.; Sadighian, S.; Khoeini, F.; Naghibi, M.; Hamidi, M. The impact of polymer coatings on magnetite nanoparticles performance as MRI contrast agents: A comparative study. DARU J. Pharm. Sci. 2015, 23, 45. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, M.W.; Kanwal, F.; Batool, A.; Jamil, T.; Zia-ul-Haq, M.; Ijaz, B.; Huang, Q.; Ullah, Z. Polymer-coated CoFe2O4 nanoassemblies as biocompatible magnetic nanocarriers for anticancer drug delivery. J. Mater. Sci. 2017, 52, 9282–9293. [Google Scholar] [CrossRef]
- Naicker, K.; Ariatti, M.; Singh, M. Active targeting of asialoglycoprotein receptor using sterically lipoplexes. Eur. J. Lipid Sci. Technol. 2016, 118, 1730–1742. [Google Scholar] [CrossRef]
- Pillay, N.S.; Daniels, A.; Singh, M. Folate-Targeted Transgenic Activity of Dendrimer Functionalized Selenium Nanoparticles in vitro. Int. J. Mol. Sci. 2020, 21, 7177. [Google Scholar] [CrossRef]
- Liu, K.; Liu, P.C.; Liu, R.; Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. 2015, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Maiyo, F.; Moodley, R.; Singh, M. Cytotoxicity, antioxidant and apoptosis studies of Quercetin-3-O-glucoside and 4-(β-D-Glucopyranosyl-1→4-α-L-Rhamnopyranosyloxy)-benzyl isothiocyanate from Moringa oleifera. Anticancer Agents Med. Chem. 2016, 16, 648–656. [Google Scholar] [CrossRef] [PubMed]








| Ferrite NPs | Crystalline Size (D) (nm) | Lattice Parameter (Å) | Lattice Strain |
|---|---|---|---|
| MgFe2O4 | 18.38 | 8.348 | 0.0071 |
| CHI-MgFe2O4 | 20.75 | 8.332 | 0.0063 |
| PVA-MgFe2O4 | 19.86 | 8.330 | 0.0069 |
| PEG-MgFe2O4 | 24.44 | 8.346 | 0.0059 |
| Figure | Coercivity (HC) (KOe) | Saturation Magnetization (MS) (emu/g) |
|---|---|---|
| MgFe2O4 | 3.24 | 55.900 |
| CHI-MgFe2O4 | 8.48 | 24.877 |
| PVA-MgFe2O4 | 3.58 | 52.408 |
| PEG-MgFe2O4 | 3.89 | 53.913 |
| Element | (Wt%) | |||
|---|---|---|---|---|
| (a) MgFe2O4 | (b) CHI-MgFe2O4 | (c) PVA-MgFe2O4 | (d) PEG-MgFe2O4 | |
| C | 9.11 | 21.23 | 8.26 | 8.37 |
| O | 39.10 | 51.92 | 34.89 | 30.89 |
| Mg | 3.72 | 2.33 | 2.41 | 2.39 |
| Fe | 48.07 | 24.52 | 54.44 | 58.35 |
| MNPs/DOX-MNPs | TEM Particle Size (nm) | Hydrodynamic Size (Mean ± Standard Error) | Zeta Potential (Mean ± Standard Error) | Polydispersity Index (PDI) |
|---|---|---|---|---|
| MgFe2O4 | 18.38 ± 1.3 | 91.5 ± 15.3 nm | −6.3 ± 1.2 mV | 0.028 |
| CHI-MgFe2O4 | 21.00 ± 0.9 | 116.7 ± 18.3 nm | −11.5 ± 0.3 mV | 0.025 |
| PVA-MgFe2O4 | 19.15 ± 1.2 | 99.7 ± 4.9 nm | −57.0 ± 0.0 mV | 0.0024 |
| PEG-MgFe2O4 | 23.28 ± 2.1 | 139.4 ± 21.0 nm | −27.1 ± 3.6 mV | 0.023 |
| DOX-CHI-MgFe2O4 | 16.24 ± 0.7 | 78.9 ± 4.5 nm | −21.8 ± 0.2 mV | 0.0033 |
| DOX-PVA-MgFe2O4 | 17.65 ± 0.5 | 87.2 ± 11.3 nm | −25.2 ± 0.4 mV | 0.017 |
| DOX-PEG-MgFe2O4 | 20.86 ± 1.3 | 98.8 ± 4.3 nm | −27.3 ± 3.6 mV | 0.0019 |
| Drug Nanocomplexes | ||||
|---|---|---|---|---|
| Cells | DOX | DOX-CHI-MgFe2O4 | DOX-PVA-MgFe2O4 | DOX-PEG-MgFe2O4 |
| HEK293 | 39.98 ± 0.3 | 18.2 ± 0.5 | 5.6 ± 0.1 | 125.9 ± 1.2 |
| Caco-2 | 67.61 ± 1.1 | 11.75 ± 0.3 | 12.3 ± 0.2 | 7.94 ± 0.5 |
| SKBR-3 | 15.85 ± 0.4 | 9.12 ± 0.3 | 3.63 ± 0.06 | 14.13 ± 0.9 |
| Drug Nanocomplexes | |||
|---|---|---|---|
| Cells | DOX-CHI-MgFe2O4 | DOX-PVA-MgFe2O4 | DOX-PEG-MgFe2O4 |
| HEK293 | 18.2 ± 0.3 | 2.95 ± 0.07 | 100.64 ± 1.1 |
| Caco-2 | 9.77 ± 0.1 | 6.31 ± 0.09 | 6.28 ± 0.08 |
| SKBR-3 | 7.59 ± 0.07 | 1.897 ± 0.01 | 11.22 ± 0.9 |
| Drug Nanocomplexes | ||||
|---|---|---|---|---|
| Cells | DOX | DOX-CHI-MgFe2O4 | DOX-PVA-MgFe2O4 | DOX-PEG-MgFe2O4 |
| HEK293 | 0.38 ± 0.004 | 0.44 ± 0.002 | 0.41 ± 0.003 | 0.47 ± 0.001 |
| Caco-2 | 0.4 ± 0.001 | 0.46 ± 0.002 | 0.5 ± 0.004 | 0.7 ± 0.012 |
| SKBR-3 | 0.47 ± 0.003 | 0.5 ± 0.004 | 0.58 ± 0.004 | 0.69 ± 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramnandan, D.; Mokhosi, S.; Daniels, A.; Singh, M. Chitosan, Polyethylene Glycol and Polyvinyl Alcohol Modified MgFe2O4 Ferrite Magnetic Nanoparticles in Doxorubicin Delivery: A Comparative Study In Vitro. Molecules 2021, 26, 3893. https://doi.org/10.3390/molecules26133893
Ramnandan D, Mokhosi S, Daniels A, Singh M. Chitosan, Polyethylene Glycol and Polyvinyl Alcohol Modified MgFe2O4 Ferrite Magnetic Nanoparticles in Doxorubicin Delivery: A Comparative Study In Vitro. Molecules. 2021; 26(13):3893. https://doi.org/10.3390/molecules26133893
Chicago/Turabian StyleRamnandan, Deevak, Seipati Mokhosi, Aliscia Daniels, and Moganavelli Singh. 2021. "Chitosan, Polyethylene Glycol and Polyvinyl Alcohol Modified MgFe2O4 Ferrite Magnetic Nanoparticles in Doxorubicin Delivery: A Comparative Study In Vitro" Molecules 26, no. 13: 3893. https://doi.org/10.3390/molecules26133893
APA StyleRamnandan, D., Mokhosi, S., Daniels, A., & Singh, M. (2021). Chitosan, Polyethylene Glycol and Polyvinyl Alcohol Modified MgFe2O4 Ferrite Magnetic Nanoparticles in Doxorubicin Delivery: A Comparative Study In Vitro. Molecules, 26(13), 3893. https://doi.org/10.3390/molecules26133893