Development and Characterization of Functional Starch-Based Films Incorporating Free or Microencapsulated Spent Black Tea Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fourier Transform Infrared (FTIR) Analysis of Films and SBT Extract Powders
2.2. Morphology of Control and Active Films
2.3. Tensile Properties, Thickness, Young’s Modulus, and Viscosity of Film-Forming Solutions
2.4. Water Vapour Transmission Rate
2.5. Light Transmission and Transparency of Films
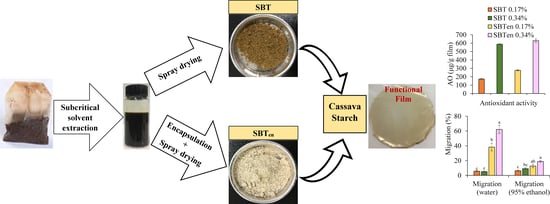
2.6. Antioxidant Content of Films and Their Migration into Food Simulants
2.7. Effect of Antioxidant Activity on Preventing Lipid Oxidation
3. Materials and Methods
3.1. Materials
3.2. Preparation of SBT Powders
3.2.1. Preparation of SBT
3.2.2. Extraction and Encapsulation of SBT Polyphenols
3.3. Preparation of the Films
3.4. Measurement of Viscosity of FFD
3.5. Characterization of the Films
3.5.1. Fourier Transform Infrared (FT-IR) Spectroscopy of Powders and Films
3.5.2. Scanning Electron Microscopy (SEM)
3.5.3. Film Thickness and Tensile Properties
3.5.4. Water Vapour Transmission Rate (WVTR)
3.5.5. Light Transmission
3.5.6. DPPH Radical Scavenging Assay
3.5.7. Migration Test
3.5.8. Peroxide Value (PV) of Soybean Oil
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vieira, S.A.; Zhang, G.; Decker, E.A. Biological Implications of Lipid Oxidation Products. J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. A Comparative Study between Natural and Synthetic Antioxidants: Evaluation of Their Performance after Incorporation into Biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, M.A.; Munekata, P.E.S.; Villanueva, N.D.M.; Tonin, F.G.; Baldin, J.C.; Rocha, Y.J.P.; Carvalho, L.T.; Rodrigues, I.; Trindade, M.A. The Antioxidant Capacity of Rosemary and Green Tea Extracts to Replace the Carcinogenic Antioxidant (BHA) in Chicken Burgers. J. Food Qual. 2017, 2409527. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing By-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; García-Estévez, I. Agricultural and Food Waste: Analysis, Characterization and Extraction of Bioactive Compounds and Their Possible Utilization. Foods 2020, 9, 817. [Google Scholar] [CrossRef]
- Butt, M.S.; Imran, A.; Sharif, M.K.; Ahmad, R.S.; Xiao, H.; Imran, M.; Rsool, H.A. Black Tea Polyphenols: A Mechanistic Treatise. Crit. Rev. Food Sci. Nutr. 2014, 54, 1002–1011. [Google Scholar] [CrossRef]
- Łuczaj, W.; Skrzydlewska, E. Antioxidative Properties of Black Tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, D.S.W.; Shimizu, N. Valorization of FFD by Recovery of Antioxidant Polyphenolic Compounds: Subcritical Solvent Extraction and Microencapsulation. Food Sci. Nutr. 2020, 8, 4297–4307. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of Yerba Mate Extract on the Performance of Starch Films Obtained by Extrusion and Compression Molding as Active and Smart Packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef] [PubMed]
- Valdés García, A.; Juárez Serrano, N.; Beltrán Sanahuja, A.; Garrigós, M.C. Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds. Antioxidants 2020, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An Overview of Encapsulation of Active Compounds Used in Food Products by Drying Technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Baracat, M.M.; Nakagawa, A.M.; Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; de Freitas, O. Preparation and Characterization of Microcapsules Based on Biodegradable Polymers: Pectin/Casein Complex for Controlled Drug Release Systems. AAPS PharmSciTech 2012, 13, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Nooshkam, M.; Varidi, M. Maillard Conjugate-Based Delivery Systems for the Encapsulation, Protection, and Controlled Release of Nutraceuticals and Food Bioactive Ingredients: A Review. Food Hydrocoll. 2020, 100, 105389. [Google Scholar] [CrossRef]
- Augustin, M.A.; Oliver, C.M.; Hemar, Y. Casein, Caseinates, and Milk Protein Concentrates. In Dairy Ingredients for Food Processing: Chandan/Dairy Ingredients for Food Processing, 1st ed.; Chandan, R.C., Kilara, A., Eds.; Blackwell Publishing Ltd.: Ames, IA, USA, 2011; pp. 161–178. [Google Scholar]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and Characterization of Cassava Starch Films Incorporated with Blueberry Pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef]
- Lim, W.S.; Ock, S.Y.; Park, G.D.; Lee, I.W.; Lee, M.H.; Park, H.J. Heat-Sealing Property of Cassava Starch Film Plasticized with Glycerol and Sorbitol. Food Packag. Shelf Life 2020, 26, 100556. [Google Scholar] [CrossRef]
- dos Santos Caetano, K.; Almeida Lopes, N.; Haas Costa, T.M.; Brandelli, A.; Rodrigues, E.; Hickmann Flôres, S.; Cladera-Olivera, F. Characterization of Active Biodegradable Films Based on Cassava Starch and Natural Compounds. Food Packag. Shelf Life 2018, 16, 138–147. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and Characterization of Active and Intelligent Packaging Films Based on Cassava Starch and Anthocyanins from Lycium Ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch Content Affects Physicochemical Properties of Corn and Cassava Starch-Based Films. Ind. Crops Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of Active and Intelligent Films Based on Cassava Starch and Chinese Bayberry (Myrica Rubra Sieb. et Zucc.) Anthocyanins. RSC Adv. 2019, 9, 30905–30916. [Google Scholar] [CrossRef] [Green Version]
- Brza, M.A.; Aziz, S.B.; Anuar, H.; Ali, F.; Dannoun, E.M.A.; Mohammed, S.J.; Abdulwahid, R.T.; Al-Zangana, S. Tea from the Drinking to the Synthesis of Metal Complexes and Fabrication of PVA Based Polymer Composites with Controlled Optical Band Gap. Sci. Rep. 2020, 10, 18108. [Google Scholar] [CrossRef] [PubMed]
- Moosa, A.; Ridha, A.M.; Allawi, M.H. Green Synthesis of Silver Nanoparticles Using Spent Tea Leaves Extract with Atomic Force Microscopy. Int. J. Curr. Eng. Sci. Res. 2015, 5, 3233–3241. Available online: http://inpressco.com/category/ijcet (accessed on 19 May 2020).
- Ali, A.; Bilal, M.; Khan, R.; Farooq, R.; Siddique, M. Ultrasound-Assisted Adsorption of Phenol from Aqueous Solution by Using Spent Black Tea Leaves. Environ. Sci. Pollut. Res. Int. 2018, 25, 22920–22930. [Google Scholar] [CrossRef] [PubMed]
- Rengga, W.D.P.; Yufitasari, A.; Adi, W. Synthesis of Silver Nanoparticles from Silver Nitrate Solution Using Green Tea Extract (Camelia Sinensis) as Bioreductor. J. Bahan Alam Terbarukan 2017, 6, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR Spectroscopy, Total Phenolic Content, Antioxidant Activity and Anti-Amylase Activity of Extracts and Different Tea Forms of Garcinia Schomburgkiana Leaves. Lebenson. Wiss. Technol. 2020, 134, 110005. [Google Scholar] [CrossRef]
- Ghazi, A. Extraction of Beta-Carotene from Orange Peels. Nahrung 1999, 43, 274–277. [Google Scholar] [CrossRef]
- Ren, J.-N.; Hou, Y.-Y.; Fan, G.; Zhang, L.-L.; Li, X.; Yin, K.; Pan, S.-Y. Extraction of Orange Pectin Based on the Interaction between Sodium Caseinate and Pectin. Food Chem. 2019, 283, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, M.H.; El-Shibiny, S. Preparation and Potential Applications of Casein-Polysaccharide Conjugates: A Review: Casein-Polysaccharide Conjugates. J. Sci. Food Agric. 2020, 100, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Zhou, X.; Liu, Y.; Li, X.; Mai, Y.; Liao, Y.; Liao, J. Physicochemical Stability and Antioxidant Activity of Soy Protein/Pectin/Tea Polyphenol Ternary Nanoparticles Obtained by Photocatalysis. Int. J. Biol. Macromol. 2018, 116, 1–7. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the Structural and Physical Properties, Antioxidant and Antimicrobial Activity of Pectin-Konjac Glucomannan Composite Edible Films Incorporated with Tea Polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Effect of Various Plasticizers and Concentration on the Physical, Thermal, Mechanical, and Structural Properties of Cassava-Starch-Based Films: Effect of Various Plasticizers. Starke 2017, 69, 1500366. [Google Scholar] [CrossRef]
- Nebahani, L.; Jaisingh, A. Polymer Science and Innovative Applications. In Chemical Analysis of Polymers: Materials, Techniques, and Future Developments, 1st ed.; Almaadeed, M.A.A., Ponnamma, D., Carignano, M.A., Eds.; Elsevier: Cambridge, MA, USA, 2020; pp. 69–117. [Google Scholar]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and Preparation of Active Starch Films Carrying Tea Polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef]
- Stoll, L.; Costa, T.M.H.; Jablonski, A.; Flôres, S.H.; de Oliveira Rios, A. Microencapsulation of Anthocyanins with Different Wall Materials and Its Application in Active Biodegradable Films. Food Bioproc. Tech. 2016, 9, 172–181. [Google Scholar] [CrossRef]
- Manshor, N.M.; Jai, J.; Hamzah, F.; Somwangthanaroj, A.; Ongdeesoontorn, W.T. Rheological Properties of Film Solution from Cassava Starch and Kaffir Lime Oil. J. Phys. Conf. Ser. 2019, 1349, 012045. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of Antioxidant Edible Films Based on Mung Bean Protein Enriched with Pomegranate Peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Chen, P.-H.; Kuo, T.-Y.; Kuo, J.-Y.; Tseng, Y.-P.; Wang, D.-M.; Lai, J.-Y.; Hsieh, H.-J. Novel Chitosan–Pectin Composite Membranes with Enhanced Strength, Hydrophilicity and Controllable Disintegration. Carbohydr. Polym. 2010, 82, 1236–1242. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of Refined Kenaf (Hibiscus Cannabinus L.) Seed Oil by Spray Drying Using β-Cyclodextrin/Gum Arabic/Sodium Caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Plakett, D.; Siro, I. Developments in Packaging Materials. In Emerging food Packaging Technologies: Principles and Practice, 1st Ed.; Yam, K.L., Lee, D.S., Eds.; Woodhead Publishing Limited: Philadelphia, PA, USA, 2012; pp. 237–359. [Google Scholar]
- Chakravartula, S.S.N.; Lourenço, R.V.; Balestra, F.; Bittante, A.M.Q.B.; Sobral, P.J.A.; Rosa, M.D. Influence of Pitanga (Eugenia uniflora L.) Leaf Extract and/or Natamycin on Properties of Cassava Starch/Chitosan Active Films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Massounga Bora, A.F.; Ma, S.; Li, X.; Liu, L. Application of Microencapsulation for the Safe Delivery of Green Tea Polyphenols in Food Systems: Review and Recent Advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in Antioxidant Active Food Packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Adilah, Z.A.M.; Jamilah, B.; Hanani, Z.A.N. Functional and Antioxidant Properties of Protein-Based Films Incorporated with Mango Kernel Extract for Active Packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration Analysis, Antioxidant, and Mechanical Characterization of Polypropylene-Based Active Food Packaging Films Loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Eugenol Incorporation into Thermoprocessed Starch Films Using Different Encapsulating Materials. Food Packag. Shelf Life 2019, 21, 100326. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Wang, S.; Liang, J.; Sun, Y.; Yue, P.; Gao, X. Packaging Films Formulated with Gelatin and Anthocyanins Nanocomplexes: Physical Properties, Antioxidant Activity and Its Application for Olive Oil Protection. Food Hydrocoll. 2019, 96, 617–624. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the Ability of Antioxidants to Counteract Lipid Oxidation: Existing Methods, New Trends and Challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Babaghayou, M.I.; Mourad, A.-H.I.; Lorenzo, V.; Chabira, S.F.; Sebaa, M. Anisotropy Evolution of Low Density Polyethylene Greenhouse Covering Films during Their Service Life. Polym. Test. 2018, 66, 146–154. [Google Scholar] [CrossRef]
- Akhter, R.; Masoodi, F.A.; Wani, T.A.; Rather, S.A. Functional Characterization of Biopolymer Based Composite Film: Incorporation of Natural Essential Oils and Antimicrobial Agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.P.Y.; Sarbon, N.M. Chicken Skin Gelatin Films with Tapioca Starch. Food Biosci. 2020, 35, 100589. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical Properties and Antioxidant Activity of Gelatin-Sodium Alginate Edible Films with Tea Polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]






| Film | Viscosity of FFD (mPa.s) | Thickness (mm) | Tensile Strength (MPa) | Young’s Modulus (MPa) | WVTR (g mm2/m2 24 h) |
|---|---|---|---|---|---|
| S | 195.5 ± 2.5 a | 0.1133 ± 0.0036 a | 13.43 ± 0.141 b | 224.45 ± 86.3 c | 0.61 ± 0.19 a |
| SBT 0.17% | 119.0 ± 5.0 b | 0.1002 ± 0.0120 ab | 17.54 ± 4.535 ab | 231.94 ± 63.7 c | 0.29 ± 0.04 b |
| SBT 0.34% | 104.3 ± 5.1 bc | 0.0957 ± 0.0028 ab | 25.33 ± 3.706 a | 1282.39 ± 84.4 a | 0.54 ± 0.12 a |
| SBTen 0.17% | 90.5 ± 1.5 bc | 0.0906 ± 0.0043 b | 25.17 ± 4.578 a | 955.05 ± 74.0 b | 0.38 ± 0.05 ab |
| SBTen 0.34% | 82.5 ± 3.5 c | 0.0862 ± 0.0032 b | 23.47 ± 5.301 ab | 1088.19 ± 72.34 ab | 0.52 ± 0.13 a |
| Film | Total Antioxidantμg (GAE)/g Film | Migration (Water) μg (GAE)/g Film | Migration (95% Ethanol) Μg (GAE)/gFilm |
|---|---|---|---|
| SBT 0.17% | 173.14 ± 6.88 d | 9.84 ± 3.00 c | 10.85 ± 7.22 c |
| SBT 0.34% | 587.06 ± 6.98 b | 30.03 ± 4.00 c | 53.31 ± 5.11 b |
| SBTen 0.17% | 276.13 ± 6.88 c | 105.63 ± 10.11 b | 35.08 ± 4.44 bc |
| SBTen 0.34% | 629.70 ± 20.80 a | 391.22 ± 24.40 a | 118.53 ± 5.12 a |
| Types | Polyphenols % | Starch (g) | SBT/SBTen (g) | Glycerol (g) | Water (g) | |
|---|---|---|---|---|---|---|
| Control films | S | 4.000 | - | 1.2 | 84.0 | |
| Films with SBT extract | SBT 0.17% | 0.17% | 3.981 | 0.0190 | 1.2 | 84.0 |
| SBT 0.34% | 0.34% | 3.962 | 0.0380 | 1.2 | 84.0 | |
| Films with encapsulatedSBT extract | SBTen 0.17% | 0.17% | 3.600 | 0.4000 | 1.2 | 84.0 |
| SBTen 0.34% | 0.34% | 3.200 | 0.8000 | 1.2 | 84.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajapaksha, S.W.; Shimizu, N. Development and Characterization of Functional Starch-Based Films Incorporating Free or Microencapsulated Spent Black Tea Extract. Molecules 2021, 26, 3898. https://doi.org/10.3390/molecules26133898
Rajapaksha SW, Shimizu N. Development and Characterization of Functional Starch-Based Films Incorporating Free or Microencapsulated Spent Black Tea Extract. Molecules. 2021; 26(13):3898. https://doi.org/10.3390/molecules26133898
Chicago/Turabian StyleRajapaksha, Surakshi Wimangika, and Naoto Shimizu. 2021. "Development and Characterization of Functional Starch-Based Films Incorporating Free or Microencapsulated Spent Black Tea Extract" Molecules 26, no. 13: 3898. https://doi.org/10.3390/molecules26133898
APA StyleRajapaksha, S. W., & Shimizu, N. (2021). Development and Characterization of Functional Starch-Based Films Incorporating Free or Microencapsulated Spent Black Tea Extract. Molecules, 26(13), 3898. https://doi.org/10.3390/molecules26133898