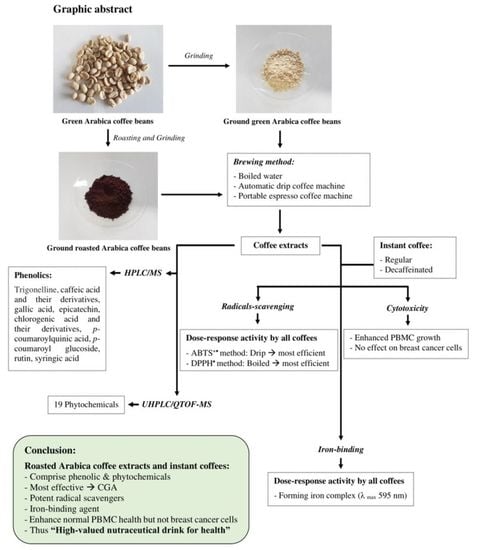
1. Introduction
Coffea arabica (Arabica) and
Coffea acanephora (Robusta) are known to be two of the most popular beverages in the world; however, Arabica coffee is more often consumed and more preferable in the global coffee market [
1,
2]. Coffee cherry husks, as well as green and roasted coffee beans, have all been processed to produce popular coffee beverages, of which roasted coffee beans are recognized as the most popular. Green coffee extract is made of unroasted green coffee beans. It is available as a dietary supplement and contains phenolic amides, as well as other phytochemicals [
3,
4]. In fact, the physical aspects, the species of the coffee bean, and the roasting and brewing processes are all important factors that influence the chemical composition of coffee beverages [
5]. These chemical compositions include caffeine (CF), chlorogenic acid (CGA), caffeic acid (CA) and Maillard reaction products (e.g., melanoidins) [
1,
5]. Besides providing an alerting effect, coffee consumption is also associated with a range of health complications such as insomnia, tremors, nausea, polyuria, diarrhea, polyphagia, hypertension and a decrease in iron absorption. All of which have been attributed to the CF and melanoidins content in coffee-based beverages [
6,
7,
8,
9]. Beneficially, coffee intake can reduce the risks associated with type 2 diabetes mellitus, Parkinson’s disease, colorectal cancer, hepatic injury, cirrhosis and hepatocellular carcinoma [
10,
11,
12,
13,
14,
15]. These benefits have been attributed to the actions of nitrogenous compounds, acids, esters and CGA [
5,
16,
17,
18]. CF (1,3,7-trimethylxanthine) is naturally found in coffee beans, cacao beans, kola nuts, guarana berries and tea leaves, of which coffee and tea are the first and second most prominent sources. The performance benefits of CF include the enhancement of mental alertness, increased levels of concentration and physical endurance, a potential reduction in fatigue and body weight and a lowering of the overall risks associated with certain metabolic syndromes [
19,
20]. CGA has three subclasses including5-
O-caffeoylquinic acid (CQA), feruloylquinic acid (FQA) and dicaffeoylquinic acid (diCQA), of which CQA is the most common and strongest antioxidant present in coffee in the form of neochlorogenic acid (3-CQA), cryptochlorogenic acid (4-CQA) and chlorogenic acid (5-CQA) [
21].
Instant coffee (regular and decaffeinated type) is a spray-dry form of coffee made from coffee extracts combined with a number of other functional ingredients (e.g., vitamins A and C, iron, inulin and oligofructose); nonetheless, the fortification of instant coffee products is necessary to improve particle size distribution, reconstitution properties, wettability and dispersibility times, as well as the overall level of satisfaction of its consumers [
22]. In the manufacturing process, decaffeinated coffee is usually prepared by a treatment with waterand an organic solvent or carbon dioxide to remove intact CF from coffee beans before they are roasted and ground.The resulting coffee beveragewill then contain 1–2% of the original CF content of the regular coffee. Even after removing CF, the decaffeinated type still contains phenolic compounds (such as CGA, CA and trigonelline) and other phytochemicals. Thesecompounds and contents are similar to those found in the regular type and are known to exertcertain biological activities of interest [
23,
24,
25,
26,
27].
Depending on the country of origin and the differing preferences of cultures and individual, coffee can be brewed by simple percolation or boiling methods, or with the use of Italian and electric coffee makers, espresso machines and French presses [
5]. However, instant coffee is made by drying prepared coffee which produces a soluble powder that can be dissolved in hot water by the consumer. Different coffee preparations result in different tastes, aromas and chemical compositions [
5,
9,
28,
29,
30]. It is likely that changes in the phenolics and CGA contents in coffee brews can affect these biological activities [
30,
31]. In contrast, degradation of 5-CQA that occurs during the roasting process does not affect antioxidant activity, whereas higher CF content has resulted in a greater degree of antioxidant activity indicating that the antioxidant activity may not depend only upon the CGA action [
32].
Many liquid chromatographic techniques have been developed for identification of the active ingredients in coffee samples. For instance, the high-performance liquid chromatography-diode array detection (HPLC-DAD) method is often used for the simultaneous quantification of CF, trigonelline, nicotinic acid, N-methylpyridinium ion, 5-CQA, and 5-hydroxymethyl furfural. The resulting values can then be compared to the specific retention times (T
R) and concentrations of the authentic standards [
33]. HPLC coupled witha mass spectrometer and a nuclear magnetic resonance spectrometer has been developed for the efficient analysis of the phenolic compounds in coffee bean extracts [
34]. Recently, a fast highly-resolved sensitive ultra-high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UHPLC-ESI-Q-TOF-MS), that involves a greater degree of informative structure elucidation and identification of the fragmentation patterns of the compounds, has been developed to identify polyphenols, alkaloids, diazines, and Maillard reaction products present in ground coffee samples [
35]. Furthermore, a fast direct form of analysis using a real time ion source coupled with high-resolution time-of-flight mass spectrometry (DART-TOF-MS) without any prior chromatographic separationhas been developed for the quantitative analysis of CF in coffee samples [
36]. In most advanced research studies, this technique can be applied for pharmaceutical, phytochemical and metabolomic analysis [
37,
38]. The present study aims to analyze the chemical compositions, assess the free-radical scavenging and iron-chelating activities and evaluate the toxicity of different coffee preparations.
3. Discussion
Coffee is understood to be the most frequently consumed beverage in most countries worldwide. The consumption of coffee can be beneficial or may actually be detrimental to human health due to the naturally occurring active compounds that are present in coffee products. Though diterpenes (e.g., cafestol and kahweol), phenolics (e.g., CGA, CA) and heterocyclic compounds present in boiled coffee (e.g., CF and melanoidins) exhibit strong antioxidant properties, these two diterpenes have been claimed to be associated with increased serum cholesterol levels [
39,
40]. In the coffee trade, coffee beans are extensively used in beverage processing and are mainly comprised of a number of functional phytochemicals and nutritional carbohydrates [
41]. Certain extraction factors can influence the quantity and activities of bioactive compounds that are present in coffee samples. These extraction factors include the use of solvents, the mass to volume ratio, acidity, time, temperature and pressure, as well as the application of microwaves or ultrasonic preparation methods and the specific type of coffee maker that may have been used [
42,
43,
44,
45]. Brewing is a key and final step in the production process of coffee drinks. The resulting drinks have been associated with coffee stoichiometry (known as total dissolved solids (TDS)), percent extraction and sweetness, and an inverse proportion to TDS. In addition, roasting is another key step in the preparation process of many of the most popular brewed coffee beverages. The roasting step can deliver a pleasant aroma while minimizing the bitterness of the final coffee beverage [
46]. In this study, we have emphasized that all data reported were based on the use of a single-type of wet-washed Arabica coffee beans to prepare coffee beverages with and without roasting. This coffee bean was selected for the production of brewed coffee using boiled water and automatic coffee makers (drip and espresso types). Thus, we have demonstrated higher coffee extraction yields by using the boiled water and drip methods over the espresso method. A previous study reported the TDS values of the espresso and drip brewed coffees (5–10% and 1.0–1.75%, respectively), and the extraction yields of the espresso coffee (approximately 14–25%) [
47]. In comparison, the extraction yields of the commercial coffee samples were found to be 81.26 ± 19.23 mg/g by methanol (100 °C), 19.12 ± 1.28 mg/g by dichloromethane (120 °C) and 1.76 ± 0.93 mg/g by
n-hexane (120 °C) [
48]. Consistent with the outcomes of our study, coffee drinks brewed with high temperatures and the cold dripping method exhibited the highest values in terms of TDS, extraction yields and the highest contents of CF, trigonelline, 4-CQA and 5-CQA, regardless of the roasting method that was used [
49]. Moreover, Angelone and coworkers have reported on the extraction yield values assayed in espresso (13.1 ± 1.3–22.8 ± 1.3%), moka (28.4 ± 1.1%), V60 (22.1 ± 0.7%), cold brew (23.3 ± 0.9%), Aeropress (20.4 ± 1.2%) and French press (18.7 ± 1.1%) coffee beverages [
50]. In roasting, galactomannan yields extracted from ground coffee with hot water were observed to increase [
51]. Importantly, the amount of ochratoxin A, a naturally occurring food-borne mycotoxin produced by
Aspergillus spp. and
Penicillium spp. that is often found in green, roasted and brewed coffee products was reduced by the use of an espresso coffee maker (49.8%), the drip-filter method (14.5%) or the moka brewing process (32.1%) [
52]. In terms of roasting coffee, CF content was not affected, CGA was degraded due to a consequence of the temperature used in brewing, CA decreased in dark roasted coffee, while melanoidins and other Maillard reaction products were developed [
53]. Importantly, the roasting of green coffee beans and the brewing of ground roasted coffee are important processes that are used to make varieties of bioactive, aromatic and popular coffee drinks.
Ultraviolet (UV) detection, derivatization spectrophotometry and gas-liquid chromatography (GLC) techniques have been developed for the simple and rapid determination of CF concentrations in beverages over a long period of time [
54]. At present, HPLC-ESI-MS and HPLC-ESI-MS-MS are known to be powerful techniques with high degrees of sensitivity and accuracy that can be used to determine the compound profiles of plant materials and natural products. Owing to the advantages of MS detection, coffee extracts were subjected to HPLC-ESI-MS identification by collision-induced dissociation mass spectrometer in order to identify certain phenolics, especially isomers. MS fragmentation patterns were observed after analysis by tandem MS spectra, while chromatographic retention times, relative hydrophobicity and bonding strength to quinic acid have been used to develop structure-diagnostic hierarchical keys for the identification of phenolic compounds. In this sense, the relative hydrophobicity of aglyconic phenolics can depend upon the substitution position of the phenolic ring and the number and identity of the residues. By using HPLC/ESI-MS/MS, we have identified at least 17 phenolic compounds, including trigonelline, CA, gallic acid, epicatechin, di-CGA, CGA, caffeoyl-
O-hexoside,
p-coumaroylquinic acid,
p-coumaroylglucoside, rutin, CGA derivative, syringic acid, CGA-glucoside, CGA-diglucoside I, CGA-diglucoside II, diCGA-glucoside, and one unknown compound in the roasted coffee extracts, whereas all of the 17 compounds and an additional three compounds, namely dimethoxycinnamic acid, caffeoylarbutin and cynaroside, were detected in the green coffee extracts. Interestingly, trigonelline, which is a bitter alkaloidal ingredient that serves as an aroma generator and is responsible for certain biological activities, was detected in both green and roasted coffee extracts. Stennert and Maier demonstrated that trigonelline was degraded gradually during the roasting stage [
55]. Similarly, CQAs, including 5-CQA,3-CQA and 4-CQA; diCQAs including 3,4-diCQA, 3,5-diCQA and 4,5-diCQA; feruloylquinic acids (FQAs) including 3-FQA, 4-FQA and 5-FQA; diFQA and
p-coumaroylquinic acids (
p-CoQAs) including 3-
p-CoQA, 4-
p-CoQA and 5-
p-CoQA isomers, were also found to be present inthe coffee samples [
56]. Likewise, CF, CQAs, diCQAs,
p-CoQAs, FQAs and caffeoylquinic acid lactone were detected in the espresso, moka, cold brewed and French Press coffee extracts [
50]. This technique has been reported to be effective in identifying certain bitter compounds, such as 1,3-bis(3′,4′-dihydroxyphenyl) butane, trans-1,3-bis(3′,4′-dihydroxyphenyl)-1-butene and eight hydroxylated phenylindanes in roasted coffee at threshold concentrations of 23–178 μM [
57], as well as in the detection of a carcinogenic furan in defatted, ground and constituted coffee preparations [
58]. In the roasting process, free amino acids and peptides existing in green coffee beans are changed into aromatic flavors, whereas polymerization and fragmentation of proteins simultaneously generate hydrogen peroxide [
59]. Unfortunately, a potential carcinogen acrylamide was also detectable in certain foods including coffee (169 ng/g) [
60].
In addition, HPLC can be employed with ESI-triple quadrupole time-of-flight mass spectrometry (HPLC-ESI-QTOF-MS) in order to provide higher resolution, faster speeds and less solvent consumption, which can lead to a rapid and sensitive characterization of certain unexpected natural products. Currently, Spreng and colleagues applied this technique for the analysis of roasted coffee and reported eleven pyrazine derivatives, of which 2-(2′,3′,4′-trihydroxybutyl)-(5/6)-methyl-pyrazine and 2,(5/6)-bis(2′,3′,4′-trihydroxybutyl)-pyrazine were the most prominent compounds [
61]. In the new findings, our UHPLC-ESI-QTOF-MS results have elucidated the presence of many phytochemicals, including 4-fluoro-L-threonine, 3-nitroperylene, cycloeudesmanesesquiterpenoids, 3,4,5-tri-CQAs, 6-gingesulfonic acid, phytosphingosine, sativanine B, sterebin E, anofinic acid, samandenone, sorbitan oleate, 2-palmitoylglycerol, citranaxanthin, 2-stearoyl glycerol, dodecanic acid and 12-methoxy-1-[(phosphonooxxy)methyl]1,2-ethanediyl ester, citronellyl butyrate, 2-[2-(4-hydroxy-3-meyhoxyphenyl)ethyl]tetrahydro-6-(4,5-dihydroxy-3-methoxyphenyl)-2H-pyran-4-ylacetate and dodemorph, in the extracts of the boiled, drip and espressogreen/roasted coffee beans. In addition, UHPLC-ESI-QTOF-MS analysis of the clinical specimens obtained from selected coffee consumers has indicated the appearance of CF, methylxanthines and methyluric acids in the plasma, along with eleven methylxanthine and methyluricacid metabolites, furan and methylfuran metabolites in the urine. These were mainly found in the form of sulfate, methyl derivatives, glucuronides and un-metabolized CQAs, FQAs, CoQAs, CA, ferulic acid and coumaric acids [
62,
63,
64].
Through the use of HPLC-DAD analysis, we have detected the presence of CF and CGA, but not CA, in the roasted Arabica coffee extracts that were prepared by boiling and with the use of coffee makers (drip and espresso) [
65]. A recent HPLC/DAD analysis of five commercial coffee samples has reported the presence of 8.35 ± 6.13 mg CF/g methanol extract [
48]. Furthermore, trigonelline, CF and CGA were detected in green coffee beans (0.65 ± 0.05, 0.97 ± 0.09 and 3.13 ± 0.33 mg/g dry weight, respectively) and roasted coffee beans (0.85 ± 0.01, 1.30 ± 0.13 and 1.00 ± 0.02 mg/g dry weight, respectively), indicating a decrease in CGA and total phytochemical contents but increases in trigonelline and CF contents during the roasting process [
66]. Likewise, trigonelline, CGA and CA were found in instant coffee samples [
24]. Due to the thermal degradation of CGA and the relative stability of the alkaloids that occur at high temperatures during the roasting process, CF and trigonelline were the main metabolites present in roasted coffee beans [
66]. During the process of roasting, CGAs and their CGA derivatives in green coffee that contribute to the acidity, astringency and bitterness of the final coffee beverages, can be isomerized and transformed to CGA lactones through a process involving water loss from quinic acid moiety and intramolecular ester bonding. This gives the coffee its flavor and determines its quality [
67]. However, the amount of CGAs and their derivatives can also be used to indicate the quality of green coffee beans and to discriminate between low-quality varieties (9.1 g CGA/100 g) and commercial ones (10.4 g/100 g) [
68]. Consistently, the phenolics found in our hot water/drip coffee extracts were mostly similar to those found in cold drip coffee extracts as determined by Angeloni et al. [
69]. Through the use of HPLC/MS in our study, the phenolic and alkaloid profiles identified for the espresso coffee extracts were not totally the same as those assayed by Aves and colleagues [
70]. However, the analyzed compounds were mostly comprised of trigonelline, CQAs, FQAs, coumaroylquinic acids, diCGAs, diFQA and caffeoylferuloylquinic acid. In the present study, when using UHPLC-ESI-QTOF-MS, 19 of 21 phytochemical compounds were detected consistently in all of the green coffee and roasted coffee extracts. Consistently, Angelino and colleagues used the UHPLC-MS-MS technique and have revealed the presence of trigonelline, CF, CGA, FQA, coumaroylquinic acids, hydroxycinnamate dimers, caffeoylshikimic acids and caffeoylquinic lactones in regular espresso coffee varieties [
71].
With regard to antioxidant activity, food processing generally affects the content and activities of persisting phytochemicals and the corresponding antioxidant capacities in functional foods. Coffee beans are the main sources of anti-oxidative compounds andrequire roasting and drying before utilization. Consequently, the two processes may give rise to significant alterations in the antioxidant compositions and properties of theresultant coffee products, while the Strecker and Maillard reactions may increasefree-radical scavenging capacities [
41]. Functional phenolic compounds contain hydroxyl components on the aglyconic phenolic ring and glyconic ring that scavenge ROS. Nonetheless, the antioxidant capacity that is related to human health benefits is dependent upon the bioavailability of the phytochemicals after consumption, which is subsequently dependent upon the soluble parts of the coffee known as the extraction yields [
17]. In accordance with this finding, all roast coffee extracts and instant coffee doses dependently scavenged ABTS
●+ and DPPH
● at different potencies depending on the coffee type and the preparation method used, in which the drip coffee extract exhibited the most efficient activity. With regard to the relevant technical factors, decaffeinated espresso coffee exerted slightly higher DPPH
● scavenging activity than regular espresso coffee (32% and 38%, respectively), which was directly related to the phenolic contents [
70]. Iwai and colleagues previously elucidated that the potency order of superoxide anion radical scavenging activity was diCGA > CA, CGA > FQA, for which the activities of the diCGA were twice as effective as those of CGA and four times as effective as those of 5-FQA [
68]. Importantly, total phenolic content, ABTS
●+- and DPPH
●-scavenging capacities of coffee samples were increased during the roasting process [
66]. More importantly, the order of antioxidant capacity of all coffee brews was espresso > mocha > plunger > drip-filtered; herein, CGAs scavenged Fremy’s salt (potassium nitrosodisulfonate) radicals, while melanoidins scavenged 2,2,6,6-tetramethyl-1-piperidin-1-oxyl radicals [
72].
Notably, the consumption of a cup of drip coffee or instant coffee resulted in approximately a 39% decrease in dietary non-heme iron. Additionally, when coffee is consumed along with a meal, ferric-ethylenediamine tetraacetic acid or ferric chloride decreases the degree of dietary iron absorption from 5.88% to 1.64% depending upon the coffee dose [
6]. For the preparation of coffee extracts, water was found to be the optimum solvent in terms of producing higher yields, better protective effects against lipid peroxidation, and effective ROS-scavenging and iron-chelating properties when compared to methanol, ethanol and
n-hexane [
73]. It was noted that certain phenolic compounds, such as CGAs, hydrolysable multi-galloyl tannin and galloylcatechin, did not condense the tannins or catechins present in the coffee utilized diol groups onto the phenolic ring that would bind iron at different affinity levels and interfere with dietary iron absorption [
74,
75]. In addition, the CGAs abundant in roasted coffee beans utilized the hydroxyl groups to bind thiol-molecules [
76]. Moreover, coffee grounds were found to adsorb divalent metal ions in the following order: Cu < Pb < Zn < Cd, whereas tea leaves exhibited a similar outcome in the opposite order [
77].
It has been reported that coffee constituents (e.g., kahweol, cafestol and CGAs) induced antioxidant-response element gene expression and activated the production of anti-oxidative enzymes in peripheral blood lymphocytes [
78]. Herein, our roasted coffee extracts significantly increased the MTT-assayed viability of normal PBMC at 48 h, but did not influence the viability of breast cancer MDA-MB-231 and MCF-7 cells at any time period. Consistently, the consumption of coffee containing cafestol and kahweol induced antioxidant enzymes by an increase of 38% of superoxide dismutase activity [
79]. Taken together, the treatment of PBMC with kahweol, cafestol and CGAs-rich roasted coffee can increase and empower mitochondrial reducing enzyme systems and subsequently intensify blue formazan products, which can result in higher percentages of cell viability. Ambiguously, a recent placebo-controlled intervention trial in healthy subjects has demonstrated that the consumption of coffee at up to 5 cups per day had no detectable beneficial or harmful effects on human health [
80]. In contrast, the biocompatible copper sulfate-oxidized nanoparticles of Arabica coffee bean extracts showed anti-proliferative activity against MCF-7 breast cancer cells [
81]. In some studies, CGAs and the natural extracts of green and roasted coffee beans could be employed as chemoprotective dietary supplements against the proliferation of Ras-dependent breast cancer MDA-MB-231 cell lines [
82]. On the other hand, trigonelline, a niacin-related natural constituent of coffee (1%), was found to stimulate MCF-7 cell growth by acting as a phytoestrogen through the mediation of the estrogen receptor [
83]. However, there is no evidence to support a relationship of either caffeinated or decaffeinated coffee intake with breast cancer risk [
84]. Despite the fact that they are the two major active flavor ingredients in coffee, CA (6 and 10 mg/plate) and CGA (19 and 28 mg/plate), have been reported for their mutagenic properties in the L5178Y mouse lymphoma TK
÷/-assay. This was possibly due to the oxidative degradation and transformation of the two compounds to hydrogen peroxide at a neutral pH in the presence of a metal ion
2+ (such as Mn
2+) [
85].Additionally, green and light roasted coffee extracts were found to promote higher inhibitory effects on the viability of PC-3 and DU-145 metastatic prostate cancer cell lines that had been assayed by the MTT test [
53]. Moreover, green coffee beans containing CGAs and their derivatives showed anti-proliferative effects against U937, KB, MCF7 and WI38-VA cancer cell lines [
68]. In controversy, diterpenes (e.g., cafestol and kahweol) are claimed to promote an increase in plasma cholesterol concentrations as a risk factor of cardiovascular diseases and type 2 diabetic mellitus [
86].