Subcritical Water Extraction of Natural Products
Abstract
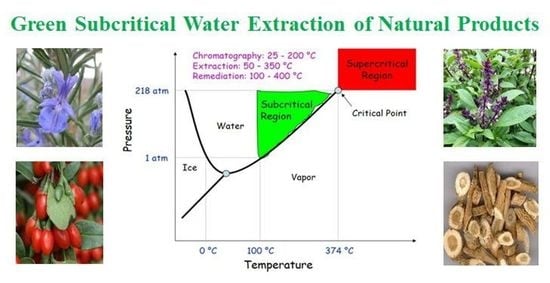
:1. Introduction
2. Sample Matrices Extracted by Subcritical Water
2.1. Plants
2.2. Food By-Products
2.3. Marine Algae
2.4. Fungi
3. SBWE Systems and Extraction Mechanism
3.1. Modes of SBWE
3.2. Online and Offline Coupling of SBWE with Liquid Chromatography
3.3. Mechanisms of Subcritical Water Extraction
4. Compounds Extracted by Subcritical Water
4.1. Flavonoids
4.2. Polyphenols
4.3. Organic Acids
4.4. Glycosides
4.5. Carbohydrates
4.6. Essential Oils
4.7. Alkaloids
4.8. Quinones
4.9. Terpenes
4.10. Lignans
4.11. Steroids
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colegate, S.M.; Molyneux, R.J. Bioactive Natural Products; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Fernández, D.P.; Goodwin, A.R.H.; Lemmon, E.W.; Sengers, J.M.H.L.; Williams, R.C. A formulation for the static permittivity of water and steam at temperatures from 238 K to 873 K at pressures up to 1200 MPa, including derivatives and Debye–Hückel coefficients. J. Phys. Chem. Ref. Data 1997, 26, 1125–1166. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y. Subcritical water chromatography: A green approach to high-temperature liquid chromatography. J. Sep. Sci. 2007, 30, 1131–1140. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Yang, Y.; Hawthorne, S.B.; Miller, D.J. Class-selective extraction of polar, moderately polar, and nonpolar organics from hydrocarbon wastes using subcritical water. Environ. Sci. Technol. 1997, 31, 430–437. [Google Scholar] [CrossRef]
- Sushkova, S.N.; Vasilyeva, G.K.; Minkina, T.M.; Mandzhieva, S.S.; Tjurina, I.G.; Kolesnikov, S.I.; Kizilkaya, R.; Askin, T. New method for benzo [a]pyrene analysis in plant material using subcritical water extraction. J. Geochem. Explor. 2014, 144, 267–272. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res. Int. 2010, 43, 1123–1129. [Google Scholar] [CrossRef]
- Islam, M.N.; Jung, H.Y.; Park, J.H. Subcritical water treatment of explosive and heavy metals co-contaminated soil: Removal of the explosive, and immobilization and risk assessment of heavy metals. J. Environ. Manag. 2015, 163, 262–269. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Technol. 2016, 46, 21–34. [Google Scholar] [CrossRef]
- Yang, R.F.; Zhao, C.; Chen, X.; Chan, S.W.; Wu, J.Y. Chemical properties and bioactivities of Goji (Lycium barbarum) polysaccharides extracted by different methods. J. Funct. Foods 2015, 17, 903–909. [Google Scholar] [CrossRef]
- Ozel, M.Z.; Gogus, F.; Lewis, A.C. Subcritical water extraction of essential oils from Thymbra spicata. Food Chem. 2003, 82, 381–386. [Google Scholar] [CrossRef]
- Xiao, S.; Xi, X.; Tang, F.; Dai, J.; Liu, J.; Lei, J.; Wang, L. Subcritical water extraction of ursolic acid from Hedyotis diffusa. Appl. Sci. 2017, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.J.; Kim, J.; Veriansyah, B.; Kim, J.D.; Lee, Y.W.; Tjandrawinata, R.R. Extraction of bioactive components from Centella asiatica using subcritical water. J. Supercrit. Fluids 2009, 48, 211–216. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, W.J.; Kim, H.J.; Kim, K.T.; Paik, H.D. Antibacterial activity of Ginseng (Panax ginseng C. A. Meyer) stems-leaves extract produced by subcritical water extraction. Int. J. Food Sci. Technol. 2013, 48, 947–953. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Chen, J.; Li, J.; Liu, S. Subcritical water extraction of alkaloids in Sophora flavescens Ait. and determination by capillary electrophoresis with field-amplified sample stacking. J. Pharmaceut. Biomed. 2012, 58, 146–151. [Google Scholar] [CrossRef]
- Zhang, G.; Chi, X. A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water. Open Chem. 2020, 18, 702–710. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Gašić, U.; Tešić, Ž.; Zengin, G.; Zeković, Z.; Đurović, S. Isolation of apigenin from subcritical water extracts: Optimization of the process. J. Supercrit. Fluids 2017, 120, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Ko, M.J.; Chung, M.S. Extraction of the flavonol quercetin from onion waste by combined treatment with intense pulsed light and subcritical water extraction. J. Clean. Prod. 2019, 231, 1192–1199. [Google Scholar] [CrossRef]
- Turner, C.; Turner, P.; Jacobson, G.; Almgren, K.; Waldebäck, M.; Sjöberg, P.; Karlsson, E.N.; Markides, K.E. Subcritical water extraction and β-glucosidase-catalyzed hyrolysis of quercetin glycosides in onion waste. Green Chem. 2006, 8, 949–959. [Google Scholar] [CrossRef]
- Esmaeelian, M.; Jahani, M.; Einafshar, S.; Feizy, J. Optimization of experimental parameters in subcritical water extraction of bioactive constituents from the saffron (Crocus sativus L.) corm based on response surface methodology. J. Food Meas. Charact. 2020, 14, 1822–1832. [Google Scholar]
- Samadi, M.; Zainal Abidin, Z.; Yoshida, H.; Yunus, R.; Biak, D.R.A. Towards higher oil yield and quality of essential oil extracted from Aquilaria malaccensis wood via the subcritical technique. Molecules 2020, 25, 3872. [Google Scholar] [CrossRef]
- Cho, Y.N.; Saravana, P.S.; David, N.; Chun, B.S. Biofunctional properties of wild cultivated and cultivated Ginseng (Panax ginseng Meyer) extracts obtained using subcritical water extraction. Sep. Sci. Technol. 2021, 56, 1370–1382. [Google Scholar] [CrossRef]
- Vardanega, R.; Carvalho, P.I.N.; Santos, D.T.; Meireles, M.A.A. Obtaining prebiotic carbohydrates and beta-ecdysone from Brazilian ginseng by subcritical water extraction. Innov. Food Sci. Emerg. Technol. 2017, 42, 73–82. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, M.J.; Chung, M.S. Subcritical water extraction of bioactive components from red ginseng (Panax ginseng C.A. Meyer). J. Supercrit. Fluids 2018, 133, 177–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Taha, A.A.; Ying, Y.; Li, X.; Chen, X.; Ma, C. Subcritical water extraction of bioactive components from ginseng root (Panax ginseng C.A. Mey). Ind. Crop. Prod. 2018, 117, 118–127. [Google Scholar] [CrossRef]
- Didar, Z. Comparative in vitro study of the biological activity and chemical composition extracts of Helicteres isora L. obtained by water and subcritical water extraction. Food Qual. Saf. 2020, 4, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Çam, M.; Yüksel, E.; Alaşalvar, H.; Başyiğit, B.; Şen, H.; Yilmaztekin, M.; Ahhmed, A.; Sağdiç, O. Simultaneous extraction of phenolics and essential oil from peppermint by pressurized hot water extraction. J. Food Sci. Technol. 2019, 56, 200–207. [Google Scholar] [CrossRef]
- Yulianto, M.; Paramita, V.; Hartati, I.; Amalia, R. Response surface methodology of pressurized liquid water extraction of curcumin from curcuma domestica val. Rasayan J. Chem. 2018, 11, 1564–1571. [Google Scholar] [CrossRef]
- Ko, M.J.; Cheigh, C.I.; Chung, M.S. Relationship analysis between flavonoids structure and subcritical water extraction (SBWE). Food Chem. 2014, 143, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Cindrić, M.; Jovanov, P.; Sakač, M.; Mandić, A.; Vidović, S. Subcritical water extraction of wild garlic (Allium ursinum L.) and process optimization by response surface methodology. J. Supercrit. Fluids 2017, 128, 79–88. [Google Scholar] [CrossRef]
- Cvetanović, A.; Zengin, G.; Zeković, Z.; Švarc-Gajić, J.; Ražić, S.; Damjanović, A.; Maškoviš, P.; Mitić, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef]
- Yu, X.M.; Zhu, P.; Zhong, Q.P.; Li, M.Y.; Ma, H.R. Subcritical water extraction of antioxidant phenolic compounds from XiLan olive fruit dreg. J. Food Sci. Technol. 2015, 52, 5012–5020. [Google Scholar] [CrossRef] [Green Version]
- Rangsriwong, P.; Rangkadilok, N.; Satayavivad, J.; Goto, M.; Shotipruk, A. Subcritical water extraction of polyphenolic compounds from Terminalia chebula Retz. fruits. Sep. Purif. Technol. 2009, 66, 51–56. [Google Scholar] [CrossRef]
- Wang, Y.; Luan, G.; Zhou, W.; Meng, J.; Wang, H.; Hu, N.; Suo, Y. Subcritical water extraction, UPLC-Triple-TOF/MS analysis and antioxidant activity of anthocyanins from Lycium ruthenicum Murr. Food Chem. 2018, 249, 119–126. [Google Scholar] [CrossRef]
- Kim, W.J.; Veriansyah, B.; Lee, Y.W.; Kim, J.; Kim, J.D. Extraction of mangiferin from Mahkota Dewa (Phaleria macrocarpa) using subcritical water. J. Ind. Eng. Chem. 2010, 16, 425–430. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Li, Z.; Liu, R.; Zhang, A.; Xiao, Z.; Ma, L.; Li, J.; Deng, S. Simultaneous extraction of oil and tea saponin from Camellia oleifera Abel. seeds under subcritical water conditions. Fuel Process. Technol. 2018, 174, 88–94. [Google Scholar] [CrossRef]
- Ravber, M.; Knez, Z.; Škerget, M. Simultaneous extraction of oil- and water-soluble phase from sunflower seeds with subcritical water. Food Chem. 2015, 166, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoez, W.; Abdelfatah, R.; Tayeb, A.; Yoshida, H. Extraction of cottonseed oil using subcritical water technology. AIChE J. 2011, 57, 2353–2359. [Google Scholar] [CrossRef]
- Ma, X.; Jing, J.; Wang, J.; Xu, J.; Hu, Z. Extraction of low methoxyl pectin from fresh sunflower heads by subcritical water extraction. ACS Omega 2020, 5, 15095–15104. [Google Scholar] [CrossRef]
- Kanmaz, E.Ö. Subcritical water extraction of phenolic compounds from flaxseed meal sticks using accelerated solvent extractor (ASE). Eur. Food Res. Technol. 2013, 238, 85–91. [Google Scholar] [CrossRef]
- Bodoira, R.; Velez, A.; Andreatta, A.E.; Martinez, M. Extraction of bioactive compounds from sesame (Sesamum indicum L.) defatted seeds using water and ethanol under sub-critical conditions. Food Chem. 2017, 237, 114–120. [Google Scholar] [CrossRef]
- Özkaynak, E.; Ova, G. The effective parameters for subcritical water extraction of SDG lignan from flaxseed (Linum usitatissimum L.) using accelerated solvent extractor. Eur. Food Res. Technol. 2013, 237, 159–166. [Google Scholar] [CrossRef]
- Hiep, N.T.; Duong, H.T.; Anh, D.T.; Nguyen, N.H.; Thai, D.Q.; Linh, D.; Anh, V.T.H.; Khoi, N.M. Subcritical water extraction of epigallocatechin gallate from Camellia sinensis and optimization study using response surface methodology. Processes 2020, 8, 1028. [Google Scholar] [CrossRef]
- Lekar, A.V.; Filonova, O.V.; Borisenko, S.N.; Maksimenko, E.V.; Vetrova, E.V.; Borisenko, N.I.; Minkin, V.I. Subcritical water extraction of chlorogenic acid from green coffee beans. Russ. J. Phys. Chem. B 2016, 9, 1043–1047. [Google Scholar] [CrossRef]
- He, C.; Du, H.; Tan, C.; Chen, Z.; Chen, Z.; Yin, F.; Xu, Y.; Liu, X. Semi-continuous pressurized hot water extraction of black tea. J. Food Eng. 2018, 227, 30–41. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Marin, F.R.; Herrero, M.; Señorans, F.J.; Reglero, G.; Cifuentes, A.; Ibáñez, E. Subcritical water extraction of nutraceuticals with antioxidant activity from oregano. chemical and functional characterization. J. Pharm. Biomed. Anal. 2006, 41, 1560–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Kayan, B.; Bozer, N.; Pate, B.; Baker, C.; Gizir, A.M. Terpene degradation and extraction from basil and oregano leaves using subcritical water. J. Chromatogr. A 2007, 1152, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Zengin, G.; Cvetanoviá, A. Chemical and bioactivity screening of subcritical water extracts of chokeberry (Aronia melanocarpa) stems. J. Pharm. Biomed. Anal. 2019, 164, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Maróstica, M.R.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Ho, B.C.H.; Kamal, S.M.M.; Danquah, M.K.; Harun, R. Optimization of subcritical water extraction (SBWE) of lipid and eicosapentaenoic acid (EPA) from nannochloropsis gaditana. BioMed Res. Int. 2018, 2018, 8273581. [Google Scholar] [CrossRef] [Green Version]
- Vo Dinh, T.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Seíoráns, F.J.; Cifuentes, A.; Ibáñez, E. Subcritical water extraction and characterization of bioactive compounds from Haematococcus pluvialis microalga. J. Pharm. Biomed. Anal. 2010, 51, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikari, F.; Han, J.; Wang, Y.; Ao, W. Synergized subcritical-ultrasound assisted aqueous two-phase extraction, purification, and characterization of Lentinus edodes polysaccharides. Process Biochem. 2020, 95, 297–306. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Gu, J.; Ji, C.; Duan, Y.; Zhang, H. Effects of subcritical water extraction microenvironment on the structure and biological activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2019, 123, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Qin, W.; Qin, P.; Zhang, H.; Duan, Y. Ultrasonic-enhanced subcritical water extraction of polysaccharides by two steps and its characterization from Lentinus edodes. Int. J. Biol. Macromol. 2018, 118, 2269–2277. [Google Scholar] [CrossRef]
- Morales, D.; Smiderle, F.R.; Villalva, M.; Abreu, H.; Rico, C.; Santoyo, S.; Iacomini, M.; Soler-Rivas, C. Testing the effect of combining innovative extraction technologies on the biological activities of obtained β-glucan-enriched fractions from Lentinula edodes. J. Funct. Foods 2019, 60, 103446. [Google Scholar] [CrossRef]
- Yang, L.; Qu, H.; Mao, G.; Zhao, T.; Li, F.; Zhu, B.; Zhang, B.; Wu, X. Optimization of subcritical water extraction of polysaccharides from Grifola frondosa using response surface methodology. Pharmacogn. Mag. 2013, 9, 120–129. [Google Scholar]
- Zhang, J.; Wen, C.; Chen, M.; Gu, J.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Antioxidant activities of Sagittaria sagittifolia L. polysaccharides with subcritical water extraction. Int. J. Biol. Macromol. 2019, 134, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Wen, C.; Zhou, J.; Gu, J.; Duan, Y.; Zhang, H.; Ren, X.; Ma, H. Structural characterization and immunostimulatory activity of a novel polysaccharide isolated with subcritical water from Sagittaria sagittifolia L. Int. J. Biol. Macromol. 2019, 133, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Duan, Y.; Yang, W.; Zhang, H.; Li, C.; Zhang, J. Structural elucidation and immunostimulatory activity of polysaccharide isolated by subcritical water extraction from Cordyceps militaris. Carbohydr. Polym. 2017, 157, 794–802. [Google Scholar] [CrossRef]
- Deshpande, G.V.; Holder, G.D.; Bishop, A.A.; Gopal, J.; Wender, I. Extraction of coal using supercritical water. Fuel 1984, 63, 956–960. [Google Scholar] [CrossRef]
- Calderon, N.B.J. Extraction of oil sand bitumens with supercritical water. Fuel Process. Technol. 1990, 25, 33–44. [Google Scholar]
- Helling, R.K.; Tester, J.W. Oxidation kinetics of carbon monoxide in supercritical water. Energy Fuels 1987, 1, 417–423. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Yang, Y.; Miller, D.J. Extraction of organic pollutants from environmental solids with sub- and supercritical water. Anal. Chem. 1994, 66, 2912–2920. [Google Scholar] [CrossRef]
- Yang, Y.; Bowadt, S.; Hawthorne, S.B.; Miller, D.J. Subcritical water extraction of polychlorinated biphenyls from soil and sediment. Anal. Chem. 1995, 67, 4571–4576. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y. Subcritical water extraction coupled to high-performance liquid chromatography. Anal. Chem. 1999, 71, 1491–1495. [Google Scholar]
- Li, B.; Gan, Y.; Eaton, C.D.; He, P.; Jones, A.D. On-line coupling of subcritical water extraction with high-performance liquid chromatography via solid-phase trapping. J. Chromatogr. A 2000, 873, 175–184. [Google Scholar] [CrossRef]
- Lamm, L.; Yang, Y. Off-line coupling of subcritical water extraction with subcritical water chromatography via a sorbent trap and thermal desorption. Anal. Chem. 2003, 75, 2237–2242. [Google Scholar] [CrossRef]
- Chen, H.M.; Fu, X.; Luo, Z.G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310. [Google Scholar] [CrossRef]
- Yang, Z.; Uhler, B.; Lipkie, T. Microwave-assisted subcritical water extraction of steviol glycosides from stevia rebaudiana leaves. Nat. Prod. Commun. 2019, 14, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Espley, R.V.; Butts, C.A.; Laing, W.A.; Martell, S.; Smith, H.; McGhie, T.K.; Zhang, J.; Paturi, G.; Hedderley, D.; Bovy, A.G.; et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J. Nutr. 2014, 144, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Ko, M.J.; Kwon, H.L.; Chung, M.S. Pilot-scale subcritical water extraction of flavonoids from satsuma mandarin (Citrus unshiu Markovich) peel. Innov. Food Sci. Emerg. Technol. 2016, 38, 175–181. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, K.T.; Kim, H.J.; Chung, M.S.; Chang, P.S.; Park, H.; Pai, H.D. Antioxidant activities of onion (Allium cepa L.) peel extracts produced by ethanol, hot water, and subcritical water extraction. Food Sci. Biotechnol. 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Kumar, M.S.; Dutta, R.; Prasad, D.; Misra, K. Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chem. 2011, 127, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Vladić, J.; Jakovljević, M.; Molnar, M.; Vidović, S.; Tomić, M.; Drinić, Z.; Jokić, S. Valorization of yarrow (Achillea millefolium L.) by-product through application of subcritical water extraction. Molecules 2020, 25, 1878. [Google Scholar] [CrossRef] [Green Version]
- Zabidi, N.A.; Ishak, N.A.; Hamid, M.; Ashari, S.E. Subcritical water extraction of antioxidants from Curculigo latifolia root. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, H.J.; Ko, M.J.; Chung, M.S. Recovery of hesperidin and narirutin from waste citrus unshiu peel using subcritical water extraction aided by pulsed electric field treatment. Food Sci. Biotechnol. 2021, 30, 217–226. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Pendleton, P.; Chun, B.S. Optimization and kinetics modeling of okara isoflavones extraction using subcritical water. Food Chem. 2019, 295, 613–621. [Google Scholar] [CrossRef]
- Ko, M.J.; Cheigh, C.I.; Cho, S.W.; Chung, M.S. Subcritical water extraction of flavonol quercetin from onion skin. J. Food Eng. 2011, 102, 327–333. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Li, H.; Xue, F.; Han, S.; Wang, L.; Cheng, Y.; Wang, X. Extraction of isoflavones from Puerariae lobata using subcritical water. RSC Adv. 2018, 8, 22652–22658. [Google Scholar] [CrossRef] [Green Version]
- Zeković, Z.; Vidović, S.; Vladić, J.; Radosavljević, R.; Cvejin, A.; Elgndi, M.A.; Pavlić, B. Optimization of subcritical water extraction of antioxidants from Coriandrum sativum seeds by response surface methodology. J. Supercrit. Fluids 2014, 95, 560–566. [Google Scholar] [CrossRef]
- Kim, D.S.; Lim, S.B. Kinetic study of subcritical water extraction of flavonoids from citrus unshiu peel. Sep. Purif. Technol. 2020, 250, 117259. [Google Scholar] [CrossRef]
- Ko, M.J.; Lee, J.H.; Nam, H.H.; Chung, M.S. Subcritical water extraction of phytochemicals from Phlomis umbrosa Turcz. Innov. Food Sci. Emerg. Technol. 2017, 42, 1–7. [Google Scholar] [CrossRef]
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process. 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Cheng, Y.; Qu, S.; Wang, Z.; Xue, F.; Li, F. Controlled extraction of flavonoids from Radix Scutellariae by subcritical water. Clean Soil Air Water 2016, 44, 299–303. [Google Scholar] [CrossRef]
- Kim, J.W.; Nagaoka, T.; Ishida, Y.; Hasegawa, T.; Kitagawa, K.; Lee, S.C. Subcritical water extraction of nutraceutical compounds from citrus pomaces. Sep. Sci. Technol. 2009, 44, 2598–2608. [Google Scholar] [CrossRef]
- Kim, D.S.; Lim, S.B. Semi-continuous subcritical water extraction of flavonoids from Citrus unshiu peel: Their antioxidant and enzyme inhibitory activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef]
- Cheigh, C.I.; Chung, E.Y.; Chung, M.S. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477. [Google Scholar] [CrossRef]
- Ho, T.C.; Chun, B.S. Extraction of bioactive compounds from pseuderanthemum palatiferum (nees) radlk. using subcritical water and conventional solvents: A comparison study. J. Food Sci. 2019, 84, 1201–1207. [Google Scholar] [CrossRef]
- Fan, R.; Xiang, J.; Li, N.; Jiang, X.; Gao, Y. Impact of extraction parameters on chemical composition and antioxidant activity of bioactive compounds from Chinese licorice (Glycyrrhiza uralensis Fisch.) by subcritical water. Sep. Sci. Technol. 2015, 51, 609–621. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Jiang, J.; Yuan, F.; Gao, Y. Subcritical water extraction and antioxidant activity evaluation with on-line HPLC-ABTS(·+) assay of phenolic compounds from marigold (Tagetes erecta L.) flower residues. J. Food Sci. Technol. 2015, 52, 3803–3811. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Ismail, M.; Baroutian, S.; Farid, M. Effect of subcritical water on the extraction of bioactive compounds from carrot leaves. Food Bioprocess Technol. 2018, 11, 1895–1903. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques: Perspective of using superheated water for isolation of biologically active compounds. Ind. Crop. Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Platonov, I.A.; Nikitchenko, N.V.; Onuchak, L.A.; Arutyunov, Y.I.; Kurkin, V.A.; Smirnov, P.V. Subcritical water extraction of biologically active substances from milk thistle seed (Silybum murianum L.). Russ. J. Phys. Chem. B 2011, 4, 1211–1216. [Google Scholar] [CrossRef]
- Vidović, S.; Nastić, N.; Gavarić, A.; Cindrić, M.; Vladić, J. Development of green extraction process to produce antioxidant-rich extracts from purple coneflower. Sep. Sci. Technol. 2018, 54, 1174–1181. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Mendiola, J.A.; Arranz, E.; Ruíz-Rodríguez, A.; Reglero, G.; Ibáñez, E.; Marín, F.R. Highly isoxanthohumol enriched hop extract obtained by pressurized hot water extraction (PHWE). Chemical and functional characterization. Innov. Food Sci. Emerg. Technol. 2012, 16, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Essien, S.; Young, B.; Baroutian, S. Subcritical water extraction for selective recovery of phenolic bioactives from kānuka leaves. J. Supercrit. Fluids 2020, 158, 104721. [Google Scholar] [CrossRef]
- Shaddel, R.; Maskooki, A.; Haddad-Khodaparast, M.H.; Azadmard-Damirchi, S.; Mohamadi, M.; Fathi-Achachlouei, B. Optimization of extraction process of bioactive compounds from Bene hull using subcritical water. Food Sci. Biotechnol. 2014, 23, 1459–1468. [Google Scholar] [CrossRef]
- Mašković, P.; Veličković, V.; Mitić, M.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Vujić, J. Summer savory extracts prepared by novel extraction methods resulted in enhanced biological activity. Ind. Crop. Prod. 2017, 109, 875–881. [Google Scholar] [CrossRef]
- Zeković, Z.; Cvetanović, A.; Švarc-Gajić, J.; Gorjanović, S.; Sužnjević, D.; Mašković, P.; Savić, S.; Radojković, M.; Đurović, S. Chemical and biological screening of stinging nettle leaves extracts obtained by modern extraction techniques. Ind. Crop. Prod. 2017, 108, 423–430. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Platonov, I.A.; Kurkin, V.A.; Afanasyeva, P.V.; Novikova, E.A.; Mukhanova, I.M. Evaluation of the extraction efficiency of biologically active compounds from chamomile flowers (Chamomilla recutita R.) grown in the Samara region by extractants in the subcritical state. Russ. J. Phys. Chem. B 2019, 12, 1212–1224. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Pendleton, P.; Sivagnanam, S.P.; Park, J.S.; Chun, B.S. Subcritical water enhances hydrolytic conversions of isoflavones and recovery of phenolic antioxidants from soybean byproducts (okara). J. Ind. Eng. Chem. 2019, 80, 696–703. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Mariotti-Celis, M.S.; Martinez-Cifuentes, M.; Perez-Correa, J.R. Glycerol as alternative co-solvent for water extraction of polyphenols from carmenere pomace: Hot pressurized liquid extraction and computational chemistry calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef] [Green Version]
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Mašković, P.; Jakšić, A. Functional coffee substitute prepared from ginger by subcritical water. J. Supercrit. Fluids 2017, 128, 32–38. [Google Scholar] [CrossRef]
- Khoza, B.S.; Dubery, I.A.; Byth-Illing, H.A.; Steenkamp, P.A.; Chimuka, L.; Madala, N.E. Optimization of pressurized hot water extraction of flavonoids from Momordica foetida using UHPLC-qTOF-MS and multivariate chemometric approaches. Food Anal. Methods 2015, 9, 1480–1489. [Google Scholar] [CrossRef]
- Vladić, J.; Janković, T.; Živković, J.; Tomić, M.; Zdunić, G.; Šavikin, K.; Vidović, S. Comparative study of subcritical water and microwave-assisted extraction techniques impact on the phenolic compounds and 5-hydroxymethylfurfural content in pomegranate peel. Plant Foods Hum. Nutr. 2020, 75, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Vidović, S.; Vladić, J.; Radosavljević, R.; Cindrić, M.; Zeković, Z. Subcritical water extraction of sage (Salvia officinalis L.) by-products—Process optimization by response surface methodology. J. Supercrit. Fluids 2016, 116, 36–45. [Google Scholar] [CrossRef]
- Ersan, S.; Ustundag, O.G.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Ko, M.J.; Nam, H.H.; Chung, M.S. Conversion of 6-gingerol to 6-shogaol in ginger (Zingiber officinale) pulp and peel during subcritical water extraction. Food Chem. 2019, 270, 149–155. [Google Scholar] [CrossRef]
- Luo, X.; Cui, J.; Zhang, H.; Duan, Y. Subcritical water extraction of polyphenolic compounds from sorghum (Sorghum bicolor L.) bran and their biological activities. Food Chem. 2018, 262, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Luo, X.; Cong, J.; Zhang, H.; Ma, H.; Duan, Y. Subcritical water extraction, identification and antiproliferation ability on HepG2 of polyphenols from lotus seed epicarp. Ind. Crop. Prod. 2019, 129, 472–479. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Gašić, U.; Tešić, Z.; Zengin, G.; Mašković, P.; Mahomoodally, M.F.; Ðurović, S. Subcritical water extraction as a cutting edge technology for the extraction of bioactive compounds from chamomile: Influence of pressure on chemical composition and bioactivity of extracts. Food Chem. 2018, 266, 389–396. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Authur, D.A.; Ma, H. Ultrasound-, subcritical water- and ultrasound assisted subcritical water-derived Tartary buckwheat polyphenols show superior antioxidant activity and cytotoxicity in human liver carcinoma cells. Food Res. Int. 2020, 137, 109598. [Google Scholar] [CrossRef] [PubMed]
- Naffati, A.; Vladić, J.; Pavlić, B.; Radosavljević, R.; Gavarić, A.; Vidović, S. Recycling of filter tea industry by-products: Application of subcritical water extraction for recovery of bioactive compounds from A. uva-ursi herbal dust. J. Supercrit. Fluids 2017, 121, 1–9. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, X.; He, L.; Yan, Q.; Yuan, F.; Gao, Y. Optimization of subcritical water extraction parameters of antioxidant polyphenols from sea buckthorn (Hippophae rhamnoides L.) seed residue. J. Food Sci. Technol. 2015, 52, 1534–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Jerković, J.; Zengin, G.; Gašić, U.; Tešić, Z.; Mašković, P.; Soares, C.; Barroso, M.F.; et al. The influence of the extraction temperature on polyphenolic profiles and bioactivity of chamomile (Matricaria chamomilla L.) subcritical water extracts. Food Chem. 2019, 271, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Zhang, H.; Dzah, C.S.; Zhang, J.; Diao, C.; Ma, H.; Duan, Y. Subcritical water extraction, identification, antioxidant and antiproliferative activity of polyphenols from lotus seedpod. Sep. Purif. Technol. 2020, 236, 116217. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; AlañǴn, M.E. Implementation of subcritical water extraction with natural deep eutectic solvents for sustainable extraction of phenolic compounds from winemaking by-products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef]
- Gagić, T.; Knez, Z.; Škerget, M. Subcritical water extraction of chestnut bark and optimization of process parameters. Molecules 2020, 25, 2774. [Google Scholar] [CrossRef]
- Vladic, J.; Nastic, N.; Stanojkovic, T.; Zizak, Z.; Cakarevic, J.; Popovic, L.; Vidovic, S. Subcritical water for recovery of polyphenols from comfrey root and biological activities of extracts. Acta Chim. Slov. 2019, 66, 473–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Process 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative phenolics obtained from spent coffee grounds (Coffea arabica L.) by subcritical water extraction. Ind. Crop. Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Najafpour-Darzi, G.; Rahimnejad, M.; Moghadamnia, A.A.; Kiamahalleh, M.V. High performance curcumin subcritical water extraction from turmeric (Curcuma longa L.). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1022, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Mottahedin, P.; Asl, A.H.; Khajenoori, M. Extraction of curcumin and essential oil from Curcuma longa L. by subcritical water via response surface methodology. J. Food Process. Preserv. 2017, 41, e13095. [Google Scholar] [CrossRef]
- Kwon, H.; Chung, M. Pilot-scale subcritical solvent extraction of curcuminoids from Curcuma long L. Food Chem. 2015, 185, 58–64. [Google Scholar] [CrossRef]
- Euterpio, M.A.; Cavaliere, C.; Capriotti, A.L.; Crescenzi, C. Extending the applicability of pressurized hot water extraction to compounds exhibiting limited water solubility by pH control: Curcumin from the turmeric rhizome. Anal. Bioanal. Chem. 2011, 401, 2977–2985. [Google Scholar] [CrossRef]
- Budrat, P.; Shotipruk, A. Enhanced recovery of phenolic compounds from bitter melon (Momordica charantia) by subcritical water extraction. Sep. Purif. Technol. 2009, 66, 125–129. [Google Scholar] [CrossRef]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Barroso, M.F.; Soares, C.; Moreira, M.M.; Morais, S.; Mašković, P.; Srček, V.G.; Slivac, I.; et al. Subcritical water extraction as an environmentally-friendly technique to recover bioactive compounds from traditional Serbian medicinal plants. Ind. Crop. Prod. 2018, 111, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive compounds of sweet and sour cherry stems obtained by subcritical water extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Gagić, T.; Knez, Ž.; Škerget, M. Hydrothermal hydrolysis of sweet chestnut (Castanea sativa) tannins. J. Serb. Chem. Soc. 2020, 85, 867–881. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.P.; Saldaña, M.D.A. Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Kheirkhah, H.; Baroutian, S.; Quek, S.Y. Evaluation of bioactive compounds extracted from Hayward kiwifruit pomace by subcritical water extraction. Food Bioprod. Process. 2019, 115, 143–153. [Google Scholar] [CrossRef]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.S. Characterization of functional materials derived from tropical red seaweed Hypnea musciformis produced by subcritical water extraction systems. J. Appl. Psychol. 2019, 31, 2517–2528. [Google Scholar] [CrossRef]
- Rodrigues, L.G.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Linares, I.B.; Mašković, P. Characterisation of ginger extracts obtained by subcritical water. J. Supercrit. Fluids 2017, 123, 92–100. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Subcritical water technology for extraction of phenolic compounds from Chlorella sp. microalgae and assessment on its antioxidant activity. Molecules 2017, 22, 1092. [Google Scholar] [CrossRef] [Green Version]
- Dorosh, O.; Moreira, M.M.; Pinto, D.; Freire, C.; Costa, P.; Rodrigues, F.; Delerue-Matos, C. Evaluation of the extraction temperature influence on polyphenolic profiles of vine-canes (Vitis vinifera) subcritical water extracts. Foods 2020, 9, 872. [Google Scholar] [CrossRef]
- Cha, J.; Kim, C.T.; Kim, T.E.; Cho, Y.J. Optimization of subcritical extraction process for cinnamon (Cinnamomum Cassia Blume) using response surface methodology. Food Sci. Biotechnol. 2019, 28, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, X.; Xu, H.; Xu, C.; Yuan, F.; Knez, Ž.; Novak, Z.; Gao, Y. Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC-ABTS+ assay. Food Bioprod. Process. 2012, 90, 215–223. [Google Scholar] [CrossRef]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Morais, S.; Barroso, M.F.; Moreira, M.M. Subcritical water extraction of antioxidants from mountain germander (Teucrium montanum L.). J. Supercrit. Fluids 2018, 138, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Jiang, Y.; Zhang, L.; Zhou, J.; Yu, Y.; Zhang, S.; Zhou, Y. Green and efficient extraction of total glucosides from Paeonia lactiflora Pall. ‘Zhongjiang’ by subcritical water extraction combined with macroporous resin enrichment. Ind. Crop. Prod. 2019, 141, 111699. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Ozturk, T.K.; Yesil-Celiktas, O.; Haznedaroglu, M.Z. Optimizing subcritical water extraction of Morus nigra L. fruits for maximization of tyrosinase inhibitory activity. J. Supercrit. Fluids 2017, 127, 15–22. [Google Scholar] [CrossRef]
- Yildiz-Ozturk, E.; Tag, O.; Yesil-Celiktas, O. Subcritical water extraction of steviol glycosides from Stevia rebaudiana leaves and characterization of the raffinate phase. J. Supercrit. Fluids 2014, 95, 422–430. [Google Scholar] [CrossRef]
- Meng, F.; Cheng, Y. Subcritical water extraction of phenolic compounds and analysis of inorganic elements from Erigeron breviscapus. ChemistrySelect 2019, 4, 7173–7180. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.M. Extraction of antioxidant compounds from different varieties of Mangifera indica leaves using green technologies. J. Supercrit. Fluids 2012, 72, 168–175. [Google Scholar] [CrossRef]
- Sarfarazi, M.; Jafari, S.M.; Rajabzadeh, G.; Feizi, J. Development of an environmentally-friendly solvent-free extraction of saffron bioactives using subcritical water. LWT 2019, 114, 108428. [Google Scholar] [CrossRef]
- Baek, J.Y.; Lee, J.M.; Lee, S.C. Extraction of nutraceutical compounds from licorice root with subcritical water. Sep. Purif. Technol. 2008, 63, 661–664. [Google Scholar] [CrossRef]
- Yilmaz-Turan, S.; Jimenez-Quero, A.; Moriana, R.; Arte, E.; Katina, K.; Vilaplana, F. Cascade extraction of proteins and feruloylated arabinoxylans from wheat bran. Food Chem. 2020, 333, 127491. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Kim, G.D.; Woo, H.C.; Chun, B.S. Subcritical water extraction of fucoidan from Saccharina japonica: Optimization, characterization and biological studies. J. Appl. Phycol. 2017, 30, 79–590. [Google Scholar] [CrossRef]
- Liew, S.Q.; Teoh, W.H.; Tan, C.K.; Yusoff, R.; Ngoh, G.C. Subcritical water extraction of low methoxyl pectin from pomelo (Citrus grandis (L.) Osbeck) peels. Int. J. Biol. Macromol. 2018, 116, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibanez, E.; Villamiel, M. Structural characterisation of pectin obtained from cacao pod husk. comparison of conventional and subcritical water extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- Ho, T.C.; Kiddane, A.T.; Sivagnanam, S.P.; Park, J.S.; Cho, Y.J.; Getachew, A.; Nguyen, T.T.; Kim, G.D.; Chun, B.S. Green extraction of polyphenolic-polysaccharide conjugates from Pseuderanthemum palatiferum (Nees) Radlk.: Chemical profile and anticoagulant activity. Int. J. Biol. Macromol. 2020, 157, 484–493. [Google Scholar] [CrossRef]
- Yan, J.K.; Wu, L.X.; Cai, W.D.; Xiao, G.S.; Duan, Y.; Zhang, H. Subcritical water extraction-based methods affect the physicochemical and functional properties of soluble dietary fibers from wheat bran. Food Chem. 2019, 298, 124987. [Google Scholar] [CrossRef]
- Chao, Z.; Ri, Y.; Tai, Q. Ultrasound-enhanced subcritical water extraction of polysaccharides from Lycium barbarum L. Sep. Purif. Technol. 2013, 120, 141–147. [Google Scholar] [CrossRef]
- Du, X.; Bai, X.; Gao, W.; Jiang, Z. Properties of soluble dietary fibre from defatted coconut flour obtained through subcritical water extraction. Int. J. Food Sci. Technol. 2019, 54, 1390–1404. [Google Scholar] [CrossRef]
- Sun, H.; Yuan, X.; Zhang, Z.; Su, X.; Shi, M. Thermal processing effects on the chemical constituent and antioxidant activity of Okara extracts using subcritical water extraction. J. Chem. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Saravana, P.S.; Cho, Y.N.; Woo, H.C.; Chun, B.S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Klinchongkon, K.; Chanthong, N.; Ruchain, K.; Khuwijitjaru, P.; Adachi, S. Effect of ethanol addition on subcritical water extraction of pectic polysaccharides from Passion fruit peel. J. Food Process. Preserv. 2017, 41, e13138. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef] [Green Version]
- Viriya-Empikul, J.W.N.; Takashi, K.; Shuji, A. Effects of temperature and flow rate on subcritical-water extraction from defatted rice bran. Food Sci. Technol. Res. 2012, 18, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S.; Mariatti, F.; Cravotto, G. Subcritical water extraction as an efficient technique to isolate biologically-active fucoidans from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 128, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Liu, W.; Qi, Q.; Hu, X.; Li, S.; Lei, J.; Rong, L. Extraction of polysaccharide from dendrobium nobile Lindl. by subcritical water extraction. ACS Omega 2019, 4, 20586–20594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordoloi, A.; Goosen, N.J. A greener alternative using subcritical water extraction to valorize the brown macroalgae Ecklonia maxima for bioactive compounds. J. Appl. Phycol. 2020, 32, 2307–2319. [Google Scholar] [CrossRef]
- Pedras, B.; Salema-Oom, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Valorization of white wine grape pomace through application of subcritical water: Analysis of extraction, hydrolysis, and biological activity of the extracts obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Pedras, B.M.; Nascimento, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Semi-continuous extraction/hydrolysis of spent coffee grounds with subcritical water. J. Ind. Eng. Chem. 2019, 72, 453–456. [Google Scholar] [CrossRef]
- Limsangouan, N.; Milasing, N.; Thongngam, M.; Khuwijitjaru, P.; Jittanit, W. Physical and chemical properties, antioxidant capacity, and total phenolic content of xyloglucan component in tamarind (Tamarindus indica) seed extracted using subcritical water. J. Food Process. Preserv. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- Nomura, S.; Lee, W.J.; Konishi, M.; Saitoh, T.; Murata, M.; Ohtsu, N.; Shimotori, Y.; Kohari, Y.; Nagata, Y.; Chiou, T.Y. Characteristics of Japanese mint extracts obtained by subcritical-water treatment. Food Sci. Technol. Res. 2019, 25, 695–703. [Google Scholar] [CrossRef]
- Eikani, M.H.; Golmohammad, F.; Rowshanzamir, S. Subcritical water extraction of essential oils from coriander seeds (Coriandrum sativum L.). J. Food Eng. 2007, 80, 735–740. [Google Scholar] [CrossRef]
- Zeković, Z.; Kaplan, M.; Pavlić, B.; Olgun, E.O.; Vladić, J.; Canlı, O.; Vidović, S. Chemical characterization of polyphenols and volatile fraction of coriander (Coriandrum sativum L.) extracts obtained by subcritical water extraction. Ind. Crop. Prod. 2016, 87, 54–63. [Google Scholar] [CrossRef]
- Ma, Q.; Fan, X.D.; Liu, X.C.; Qiu, T.Q.; Jiang, J.G. Ultrasound-enhanced subcritical water extraction of essential oils from Kaempferia galangal L. and their comparative antioxidant activities. Sep. Purif. Technol. 2015, 150, 73–79. [Google Scholar] [CrossRef]
- Musa, T.A.; Sanagi, M.M.; Ibrahim, W.A.W.; Ahmad, F.; Aboul-Enein, H.Y. Determination of 4-allyl resorcinol and chavibetol from piper betle leaves by subcritical water extraction combined with high-performance liquid chromatography. Food Anal. Methods 2013, 7, 893–901. [Google Scholar] [CrossRef]
- Samadi, M.; Abidin, Z.Z.; Yoshida, H.; Yunus, R.; Biak, D.R.A.; Lee, C.H.; Lok, E.H. Subcritical water extraction of essential oil from Aquilaria malaccensis leaves. Sep. Sci. Technol. 2019, 55, 2779–2798. [Google Scholar] [CrossRef]
- Fernández-Pérez, V.; Jiménez-Carmona, M.M. An approach to the static-dynamic subcritical water extraction of laurel essential oil: Comparison with conventional techniques. Analyst 2000, 125, 481–485. [Google Scholar] [CrossRef]
- Halim, N.A.A.; Abidin, Z.Z.; Siajam, S.I.; Hean, C.G.; Harun, M.R. Screening of factors influencing the yield of Citrus hystrix leaves essential oil extracted via pressurized hot water extraction based on resolution V fractional factorial design. J. Food Eng. 2020, 43, 1–13. [Google Scholar] [CrossRef]
- Pavlić, B.; Vidović, S.; Vladić, J.; Radosavljević, R.; Zeković, Z. Isolation of coriander (Coriandrum sativum L.) essential oil by green extractions versus traditional techniques. J. Supercrit. Fluids 2015, 99, 23–28. [Google Scholar]
- Filly, A.; Fabiano-Tixier, A.S.; Louis, C.; Fernandez, X.; Chemat, F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. Comptes Rendus Chim. 2016, 19, 707–717. [Google Scholar] [CrossRef]
- Liu, F.; Wan, S.Y.; Jiang, Z.; Li, S.F.; Ong, E.S.; Osorio, J.C. Determination of pyrrolizidine alkaloids in comfrey by liquid chromatography-electrospray ionization mass spectrometry. Talanta 2009, 80, 916–923. [Google Scholar] [CrossRef]
- Mokgadi, J.; Turner, C.; Torto, N. Pressurized hot water extraction of alkaloids in goldenseal. Am. J. Anal. Chem. 2013, 4, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Jokic, S.; Gagic, T.; Knez, Z.; Subaric, D.; Skerget, M. Separation of active compounds from food by-product (Cocoa shell) using subcritical water extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef] [Green Version]
- Šeremet, D.; Durgo, K.; Jokić, S.; Huđek, A.; Cebin, A.V.; Mandura, A.; Jurasović, J.; Komes, D. Valorization of banana and red beetroot peels: Determination of basic macrocomponent composition, application of novel extraction methodology and assessment of biological activity in vitro. Sustainability 2020, 12, 4539. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem. 2012, 135, 943–949. [Google Scholar] [CrossRef] [Green Version]
- Machmudah, S.; Lestari, S.D.; Widiyastuti; Wahyudiono; Kanda, H.; Winardi, S.; Goto, M. Subcritical water extraction enhancement by adding deep eutectic solvent for extracting xanthone from mangosteen pericarps. J. Supercrit. Fluids 2018, 133, 615–624. [Google Scholar] [CrossRef]
- Hashim, N.A.; Mudalip, S.K.A.; Harun, N.; Man, R.C.; Sulaiman, S.Z.; Arshad, Z.I.M.; Shaarani, S.M.; Azmir, J. Mahkota dewa Subcritical water extraction process: Experimental and molecular dynamics simulation study. Chem. Eng. Technol. 2019, 42, 1747–1756. [Google Scholar] [CrossRef]
- Yang, R.; Huang, P.; Qiu, T. Ultrasound-enhanced subcritical water extraction of naphthoquinone pigments from purple gromwell (Lithospermum erythrorhizon) to higher yield and bioactivity. Food Sci. Biotechnol. 2013, 22, 671–676. [Google Scholar] [CrossRef]
- Anekpankul, T.; Goto, M.; Sasaki, M.; Pavasant, P.; Shotipruk, A. Extraction of anti-cancer damnacanthal from root of Morinda citrifolia by subcritical water. Sep. Purif. Technol. 2007, 55, 343–349. [Google Scholar] [CrossRef]
- Shotipruk, A.; Kiatsongserm, J.; Pavasant, P.; Goto, M.; Sasaki, M. Pressurized hot water extraction of anthraquinones from the root of Morinda citrifolia. Biotechnol. Prog. 2004, 20, 1872–1875. [Google Scholar] [CrossRef] [PubMed]
- Pongnaravane, B.; Goto, M.; Sasaki, M.; Anekpankul, T.; Pavasant, P.; Shotipruk, A. Extraction of anthraquinones from root of Morinda citrifolia by pressurized hot water: Antioxidant activity of extracts. J. Supercrit. Fluids 2006, 37, 390–396. [Google Scholar] [CrossRef]
- Uzel, R.A.; Yaman, Ü.R. A novel method for food particle production using subcritical water extraction: Ganoderma mushroom as a case example. J. Supercrit. Fluids 2016, 111, 74–82. [Google Scholar] [CrossRef]
- Ko, M.J.; Nam, H.H.; Chung, M.S. Subcritical water extraction of bioactive compounds from Orostachys japonicus A. Berger (Crassulaceae). Sci. Rep. 2020, 10, 10890. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, P.; Yao, W.; Wang, J.; Wang, L.; Deng, L.; He, J.; Zhang, G.; Lei, J. Subcritical water extraction of betulinic acid from birch bark. Ind. Crop. Prod. 2015, 74, 557–565. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, G.; Chen, S. Subcritical water extraction of sesquiterpene lactones from Inula racemose. ChemistrySelect 2020, 5, 488–494. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, W.; Gao, Q.; Yu, S.; Xue, F. Enhanced extraction of bioactive components of 3,29-dibenzoylkarounidiol and polysaccharides from Semen richonsanthis using subcritical water technology. ChemistrySelect 2019, 4, 13689–13694. [Google Scholar] [CrossRef]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Extraction of pumpkin peel extract using supercritical CO2 and subcritical water technology: Enhancing oxidative stability of canola oil. J. Food Sci. Technol. 2020, 54, 2306–2315. [Google Scholar] [CrossRef]
- Falev, D.I.; Shestakov, S.L. Subcritical extraction of birch bark pentacyclic triterpenes. Russ. Chem. B 2017, 66, 875–881. [Google Scholar] [CrossRef]
- Kovacevic, D.B.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragovic-Uzelac, V.; Putnik, P. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem. 2018, 254, 150–157. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Chi, X.; Chen, S. Green and efficient extraction of podophyllotoxin from Sinopodophyllum hexandrum by optimized subcritical water extraction combined with macroporous resin enrichment. Ind. Crop. Prod. 2018, 121, 267–276. [Google Scholar] [CrossRef]
- Gabaston, J.; Leborgne, C.; Valls, J.; Renouf, E.; Richard, T.; Waffo-Teguo, P.; Mérillon, J.M. Subcritical water extraction of stilbenes from grapevine by-products: A new green chemistry approach. Ind. Crop. Prod. 2018, 126, 272–279. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Gansukh, E.; Baskar, V.; Kai, G. Subcritical water extraction of withanosides and withanolides from ashwagandha (Withania somnifera L) and their biological activities. Food Chem. Toxicol. 2019, 132, 110659. [Google Scholar] [CrossRef]
- Najjar-Tabrizi, R.; Javadi, A.; Sharifan, A.; Chew, K.W.; Lay, C.H.; Show, P.L.; Jafarizadeh-Malmiri, H.; Berenjian, A. Hydrothermally extraction of saponin from Acanthophyllum glandulosum root-Physico-chemical characteristics and antibacterial activity evaluation. Biotechnol. Rep. 2020, 27, e00507. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Balsevich, J.; Mazza, G. Pressurized low polarity water extraction of saponins from cow cockle seed. J. Food Eng. 2007, 80, 619–630. [Google Scholar] [CrossRef]










| Samples | Medicinal Parts | Compounds Extracted | Extracts Activity | Extraction Conditions | Analytical Methods | Other Extraction Methods (Solvent, Ratios of Yields) | Ref. |
|---|---|---|---|---|---|---|---|
| Panax ginseng C.A. Meyer | stem leave | TP and flavonoids | antibacterial | 110 and 165 °C, 15 min 190 °C, 10 min | TEM, UV | heating (water 95.4%; ethanol 91.3%) | [15] |
| Chamomilla matricaria L. | flowers | TP, TF, 18 polyphenolic compounds, apigenin | antioxidant, enzyme inhibitory activity | 65–210 °C, 5–60 min 1:30–1:100 g/mL | TLC, UV, HPLC-MS | [18] | |
| Allium cepa | onion wastes | quercetin-4′-glycoside, quercetin, etc. | 40–160 °C, 5 min, 5 MPa, 1–10 mm, pH 3.0–7.0 | LC-MS/MS HPLC-UV | convention (methanol and hydrochloric acid 94.3%) | [19] | |
| Crocus sativus L. | stigmas | TP, dodecane, γ-terpinene, tetradecane, etc. | antioxidant (DPPH, FRAP), antibacterial | 100–180 °C, 10–30 min, 1:10 g/mL | GC/MS, UV-vis | [21] | |
| Saururus chinensis, etc. | skin, leave, peel, etc. | quercetin, isorhamnetin, kaempferol, isoquercitrin, etc. | 10 MPa, 110–200 °C, 5–15 min | HPLC | [30] | ||
| Camellia sinensis | leaves | epigallocatechin gallate | 80–120 °C, 3–7 min, 40–60 mL/g | HPLC | convention (water 87.6%) | [44] | |
| Origanum vulgare L. | leaves | TP, flavanone, flavone, flavanol | antioxidant (DPPH, TEAC, ABTS) | 10.34 MPa, 30 or 15 min 25–200 °C | HPLC- DAD, UV | [47] | |
| orange | peels | reducing sugar, TP, pectin, hesperidin, narirutin | antioxidant (DPPH, FRAP) | 110–150 °C, 10–30 mL/min 10 MPa | HPLC, UV-vis | Soxhlet (ethanol 79.2%), shaker (ethanol 250%), UAE (ethanol 114%) | [50] |
| orange | peels | flavones, 7-hydroxyflavone | 100–150 °C, 0.5 mL/min | GC-FID | UAE (methylene chloride) | [70] | |
| Citrus unshiu Markovich | peels | rutin, naringin, hesperidin, naringenin | 0.5–14 MPa, 5–15 min, 100–190 °C | HPLC | [74] | ||
| Allium cepa L. | peels | TP, TF, quercetin | antioxidant (DPPH, TBA, FTC) | 110 and 165 °C, 15 min, p < 3.4 MPa | HPLC, UV | heating (ethanol 153%; water 45.6%) | [75] |
| Hippophae rhamnoides | leaves | TP, TF, isorhamnetin, kaempferol, quercetin | antioxidant, cytotoxicity | 25–200 °C, 15 min, 10.34 MPa | HPLC, UV, FM | maceration (water 21.3%), Soxhlet (ethanol 64.6%) | [76] |
| Allium cepa L. | peels | TP, TF, kaempferol, quercetin | antioxidant (DPPH) | 170–230 °C, 3 MPa, 30 min, pH 2–10 | HPLC, UV-vis | heating (ethanol 26.7%) | [77] |
| Achillea millefolium L. | herbal dust | TP, TF, HMF, chlorogenic acid | antioxidant (DPPH, TEAC, ABTS) | 120–200 °C, 10–30 min 0–1.5% HCl, 3 MPa | HPLC, UV-vis | [78] | |
| Curculigo latifolia | root | TP, TF, pomiferin, etc. | antioxidant (DPPH, ABTS, TEAC) | 100–200 °C, 10 MPa 30–120 min, 0.5 mL/min | LC-MS, UV | [79] | |
| Citrus unshiu | peels | hesperidin and narirutin | 110–190 °C 3–15 min | HPLC | [80] | ||
| Glycine max | okara | genistin, daidzin, genistein, daidzein | 100–200 °C, 5 min, 2–5 MPa, 10–30 g/mL | HPLC | Soxhlet (methanol, 108%) | [81] | |
| onion | skins | quercetin, quercetin-4′-glucoside | 100–190°C, 5–30 min, 9–13 MPa | HPLC | convention (methanol, 92.8%) | [82] | |
| Puerariae lobata | root | puerarin, daidzin, daidzein 3-methoxypuerarin | 100–200 °C, 15–75 min 1:10–1:25 g/mL | HPLC | reflux (ethanol 91.6%), UAE (water 95.9%) | [83] | |
| Coriandrum sativum | seeds | TP, TF | antioxidant (DPPH) | 100–200 °C, 10–30 min 3–9 MPa | UV | [84] | |
| Citrus unshiu | peels | flavanones, polymethoxy-Flavones, etc. | anticancer, cardioprotectives | 120–180 °C, 1.0–2.0 mL/min, 5.0 ± 0.1 MPa | GC, HPLC, | convention (methanol 75.0%; ethanol 41.6%; acetone 17.2%) | [85] |
| Phlomis umbrosa | whole part | TP, TF, iridoids glycosides | antioxidant (DPPH, ABTS) | 110–200 °C, 10 MPa, 1–25 min | HPLC, ESI-MS | convention (ethanol; methanol; water) | [86] |
| Actinidia deliciosa | peels | TP, TF, | antioxidant (DPPH, ABTS, FRAP) | 120–160 °C, 0–30 min, 3 MPa, pH 2–5.5 | UV-vis, pH | convention (ethanol 81.9%) | [87] |
| Scutellaria baicalensis | root | baicalin, baicalein, wogonin, wogonoside | 110–160 °C, 10–90 min, 20–100 mesh | HPLC | HRE (ethanol 93.0%) | [88] | |
| Citrus unshiu | pomaces | TP, polymethoxylated flavones, sinensetin, etc. | antioxidant (DPPH, TP) | 25–250 °C, 10–60 min, 0.1–5.0 MPa | HPLC, UV | [89] | |
| citrus unshiu | peels | hesperidin, narirutin, prunin, naringenin, sinensetin, etc. | antioxidants (DPPH, FRAP), enzyme | 145–175 °C, 15 min 5 MPa, 0.75–2.2 mL/min | HPLC | 2M HCl extraction 42.9%; 2 M NaOH extraction 38.9% | [90] |
| citrus unshiu | peels | hesperidin and narirutin | 110–200 °C, 5–20 min, 10 ± 1 MPa | HPLC, MS/MS | convention (ethanol 56.4%; methanol 35.8%; water 6.2%) | [91] | |
| palatiferum Radlk. | leaves | TP, TF, protein, saponin, sugar, apigenin, kaempferol | antioxidants (DPPH, FRAP, ABTS), | 110–270 °C, 15 min, 8 MPa 1:70 g/mL | HPLC, UV | convention (water 77.7%; methanol 32.8%), Soxhlet (ethanol 43.7%) | [92] |
| Glycyrrhiza uralensis Fisch. | root | TP, TF, liquiritin, flavanone, isoflavone | antioxidants (DPPH, ABTS) | 80–320°C, 2–100 min, 7.0 MPa, 1:30 g/mL, pH 3–11 | HPLC, MS/MS, UPLC | UAE (water 20.6%; ethanol 44.9%), MAE (water 25.6%; ethanol 63.8%) | [93] |
| Tagetes erecta L. | flower residues | TP, TF, 5-HMF, reducing sugar, free amino acids | antioxidants (DPPH, ABTS) | 80–260 °C, 15–90 min 1:20–1:60 g/mL,120 rpm | HPLC-DAD, UV | leaching (water 9.4%; methanol 69.9%; ethanol 68.8%; acetone 94.0%), UAE (water 9.9%; methanol 69.8%; ethanol 64.3%; acetone 87.6%) | [94] |
| Daucus carota | leaves | polyphenols, luteolin | 110–230 °C, 0–114 min, 4 MPa | UV, PLC | [95] | ||
| Matricaria chamomilla L. | flowers | TP, TF, apigenin-7-O-glucoside, etc. | antimicrobial, cytotoxic activity | 200 °C, 40 min, 1:50 g/mL | UHPLC, HESI- MS/MS, UV | Soxhlet (ethanol 129%), MAE (ethanol 117%), UAE (ethanol 104%) | [96] |
| Silybum murianum L | seeds | taxifolin, silychristin, silydianin, and silybin | 75–250 °C, 40–60 min, 12.5 MPa, 0.1–0.5 mm | HPLC | convention (ethanol 101%; water 43.6%) | [97] | |
| Echinacea purpurea L. | flowers | TP, TF | antioxidant (TEAC, ABTS) | 103.4–216.56 °C, 3 MPa, 5.86–34.414 min | UV-vis | [98] | |
| Humulus lupulus | pellets | TP, desmetylxanthohumol, prenylflavonoids, etc. | anti-inflammatory | 50–200 °C, 30 min, 10 MPa | HPLC, MS/MS | convention (hexane 17.2%; ethanol 105%) | [99] |
| Kunzea ericoides | leaves | TP, TF, 5-HMF, quercetin, catechin, syringic acid, etc. | antioxidant (DPPH, FRAP) | 150–210 °C, 0–40 min 15–35 g/mL, 4 MPa | HPLC, UV | convention (ethanol 37.5%) | [100] |
| Pistacia atlantica subsp. mutica | hull | TP, kaffesaure, ethyl vanillin, flavanomarein, etc. | antioxidant (DPPH), reducing power | 110–200 °C, 30–60 min, 10–50 g/mL | HPLC-DAD, UV | HWE (85 °C 42.8%) | [101] |
| Satureja hortensis L. | whole part | TP, TF, rosmarinic acid, rutin, quercetin, etc. | cytotoxic, antibacterial | 140 °C, 30 min 4 MPa, 1:20 g/mL | HPLC-PDA, UV | maceration (ethanol 57.2%), Soxhlet (ethanol 18.4%), UAE (ethanol 69.2%), MAE (ethanol 81.3%) | [102] |
| Urtica dioica L. | leaves | TP, TF, twenty-seven compounds | cytotoxic, antifungal, antimicrobial | 125 °C, 30 min, 3.5 MPa, 1:30 g/mL | UHPLC-HESI-MS/MS | UAE (water 48.5%), MAE (water 100%) | [103] |
| Chamomilla recutita R. | flowers | 2 flavonoids, 4 esters, 1 amino acid, 11 phenols, etc. | 150 or 200 °C, 5.0 ± 0.1 MPa, 1.7 mL/min, 40 min | UV, HPLC, GC-MS | [104] | ||
| Glycine max | okara | TP, gallic acid, syringic acid, ferruric acid, etc. | antioxidant (ABTS, DPPH, FRAP) | 150 °C, 4 MPa, 5–275 min 20 mg/mL | UV, HPLC | [105] | |
| Carménère grape | pomace | flavanols, stilbenes, and phenolic acids | 90–150 °C, 5 min, 10 MPa, 15–50% glycerol | UPLC-MS | [106] | ||
| Zingiber officinale | root | TP, TF, four macro- and five microelements | antioxidant (OH·, ABTS, TRP, etc.) | 80–180 °C, 1 h, 5MPa, 1:10 g/mL | UV-vis, ICP-MS | convention (water, 62.5%) | [107] |
| Momordica foetida | leaves | quercetin, kaempferol, isorhamnetin | 100–300 °C, 5 mL/s 6.9± 1.4 MPa psi | UHPLC-q-TOF-MS | [108] |
| Samples | Medicinal Parts | Compounds Extracted | Extracts Activity | Extraction Conditions | Analytical Methods | Other Extraction Methods (Solvent, Ratios of Yields) | Ref. |
|---|---|---|---|---|---|---|---|
| Allium ursinum L. | leaves | TP, TF, 5-HMF, catechin, p-cumaric, ferulic acids, etc. | antioxidant (DPPH, ABTS), Millard products | 120–200 °C, 10–30 min, 0–1.5% HCl, 1:10 g/mL | HPLC-DAD | [31] | |
| Terminalia chebula | fruits | TP, allic acid, corilagin ellagic acid | antioxidant (ABTS) | 120–220 °C, 2–4 mL/min, 4 MPa | TLC, UV, MS, NMR, HPLC | Soxhlet (water 74.5%; ethanol 46.3%), HWE (water 46.3%) | [34] |
| Lycium ruthenicum Murr. | fruits | total anthocyanin, seven anthocyanins | antioxidant (ABTS, DPPH) | 110–170 °C, 30–90 min, 1–3 min/L | HPLC, UPLC-MS | UAE (water 59.8%; methanol 81.1%) | [35] |
| Punica granatum L. | peels | TP, TF, punicalin, etc. | 100–220 °C, 5–30 min, 3.0 MPa | UV-vis, HPLC | MAE (water 121%; ethanol 146%) | [109] | |
| Castanea sativa | shells | tannins, phenolic acids, flavonoids, anthocyanins | antioxidant (DPPH, FRAP, ABTS) | 51–249 °C, 6–30 min | UV-vis, LC/ESI-MS | [110] | |
| Salvia officinalis L. | by–products | TP, TF | antioxidant (DPPH, TEAC, reducing power) | 120–220°C, 10–30 min, 3 MPa, 0–1.5% HCl | UV | maceration (water 59.9%) | [111] |
| Pistacia vera L. | hulls | gallotannin, anacardic acid, etc. | antioxidant (ABTS, FRAP) | 110–190 °C, 6.9 MPa, 4 mL/min | HPLC-ESI/MSn | UAE (methanol 83.9%)) | [112] |
| Zingiber officinale | pulp and peel | 6-gingerol, 6-shogaol | antioxidant (FRAP) | 10 MPa, 110–190 °C, 5–40 min | HPLC | convention (methanol 114%; water 77.1%) | [113] |
| Sorfhum bicolor L. | bran | TP, oligomeric procyanidins, taxifolin, taxifolin hexoside | antioxidant (DPPH, ABTS), antiproliferative | 110–190 °C, 5–40 min, 1:10–1:50 g/mL | HPLC, ESI-MS/MS | heating (water 74.9%) | [114] |
| Nelumbo nucifera | seed epicarp | TP, proanthocyanidin dimers, trimer, cyanidin, etc. | antiproliferation effect (MTT) | 100–180 °C, 5–25 min, 1:20–1:60 g/mL, 1–5‰ NaHSO3 | HPLC-ESI-MS, UV | HWE (water 33.9%) | [115] |
| German chamomile | flowers | 9 phenolic acids and derivatives | antioxidant, cytotoxic, enzyme | 100 °C, 1–9 MPa, 30 min | UHPLC-DAD, MS/MS | [116] | |
| Fagopyrum tataricum | grains | phenols, 13 phenolics, 4 flavonoids, 3 anthocyanins | antioxidant (TEAC, CAA and FRAP), cytotoxicity | 220 °C, 60 min, 5 MPa, 1:60 g/mL | HPLC-MS, UV | UAE (water 83.5%) | [117] |
| A. uva–ursi | herbal dust | TP, TF | antioxidant (DPPH, reducing power) | 120–220 °C,3 MPa, 10–30 min, 0–1.5% HCl | UV | maceration (water 38.5%; ethanol 69.5%) | [118] |
| Hippophaë rhamnoides L. | seed residue | TP, TF, proanthocyanidins | antioxidant (DPPH) | 80–180 °C, 15–90 min, 1:10–1:50 g/mL, 6 MPa | UV | convention (water 19.6%; methanol 104%; ethanol 80.0%) | [119] |
| grape (Croatina) | pomace | TP, TF | antioxidant (DPPH) | 100–140 °C, 8–15 MPa, 1–2 mL/min | UV | convention (water 5.3%; ethanol 7.87%) | [120] |
| Matricaria chamomilla L. | flowers | polyphenolic compounds, etc. | antioxidant, cytotoxic, enzyme inhibitory | 65–210 °C, 30 min, 4.5 MPa | UHPLC-ESI-MS/MS, UV | [121] | |
| Nelumbo nucifera | seedpods | TP, TF, proanthocyanidin dimer, isoquercetin, etc. | antioxidant, antiproliferative (HepG2) | 100–180 °C, 30–70 mL/g, 5–25 min, 1–6‰ NaHSO3 | UV-Vis, HPLC, ESI-MSn | HWE (water 91.4%) | [122] |
| Vitis vinifera L. | grape pomace | catechins, flavonols, tannins, proanthocyanidins, etc. | antioxidant (DPPH, ABTS) | 40–120 °C, 10 min, 10.34 MPa, 10–40% NADES | UV, HPLC-ESI-MS | [123] | |
| sweet chestnut | bark | TP, tannins, ellagic and gallic acids, ellagitannins, etc. | antioxidant (DPPH) | 150–250 °C, 10–60 min, 10–30 mL/g, 4.5 MPa | UV-Vis, HPLC | [124] | |
| Symphytum officinale | root | TP, TF | antioxidant (DPPH), enzyme inhibitory | 120–200 °C,10–30 min, 0–1.5% HCl | UV, ELISA | UAE (methanol 2.5%; ethanol 17.4%); maceration (methanol 4.4%; ethanol 29.8%) | [125] |
| Pinot Nero | grape skins | TP | 80–120 °C, 2 h,10 MPa, 2–5 mL/min | UV-Vis | [126] | ||
| Coffea arabica L. | spent coffee grounds | TP, caffeoylquinic acid, feruloylquinic acid, etc. | antioxidant (DPPH, ABTS) | 160–180 °C, 35–55 min, 14.1–26.3 g/L | HPLC-ABTS+, MS, UV | [127] | |
| Curcumalonga L. | rhizomes | curcumin, demethoxycurcumin | 120–160 °C, 6–22 min, 1–2.5 MPa | HPLC-UV, SEM | [128] | ||
| Curcuma longa L. | rhizomes | α-phellandrene, curcumin, β-caryophyllene, trans-β-farnesene, β-bisabolene, γ-curcumin, etc. | 90–150 °C, 1–4 mL/min, 2 MPa, 0.5–1.5 mm | GC/GC-MS, GC -FID | HD (80.7%), Soxhlet (n-hexane 1.2-fold) | [129] | |
| Curcuma longa L. | rhizomes | curcumin, demethoxycurcumin, bisdemethoxycurcumin | 110–150 °C, 1–10 min, 0.5–10 MPa | HPLC | convention (ethanol, 1.13-fold) | [130] | |
| Curcuma longa L. | rhizomes | curcumin, demethoxycurcumin, bisdemethoxycurcumin | 90–250 °C, pH 1.0–5.5 5.0 MPa, 0.5 mL/min | HPLC, UPLC, LC-MS | Soxhlet (acetone, 1.17-fold) | [131] |
| Samples | Medicinal Parts | Compounds Extracted | Extracts Activity | Extraction Conditions | Analytical Methods | Other Extraction Methods (Solvent, Ratios of Yields) | Ref. |
|---|---|---|---|---|---|---|---|
| Panax ginseng Meyer | root | TP, chlorogenic acid, caffeic acid, gallic acid, etc. | antioxidant (DPPH, ABTS, FRAP, HRS) | 100–240 °C, 15 min, 4–9 MPa, 200 rpm | HPLC, UV | [23] | |
| Helicteres isora L. | hexadecanoic acid, octadecnoic acid, heptadecen-8-carbonic acid etc. | antibiofilm, antioxidant, antimicrobial, antienzymatic | 160 °C, 30 min, 1 MPa, 1: 30 g/mL | GC-MS, UV | [27] | ||
| XiLan olive fruit | olive dreg | TP, chlorogenic acid, gallic acid, syringic acid, etc. | antioxidant (ABTS, DPPH, reducing power) | 100–180 °C, 5–60 min, 1:20–1:60 g/mL | LC-MS-IT-TOF, UV | convention (methanol 3.2%; ethanol 0.6%; DMK 0.9%) | [33] |
| Camellia oleifera Abel. | seeds | free fatty acids (palmitic acid, stearate, oleic acid, etc.), tea saponin | antioxidant (DPPH) | 60–160 °C,2–7 MPa, 5–60 min, 1:3–1:25 g/mL | GC-MS, FT-IR | Soxhlet (petroleum ether 100%), cold pressed (100%) | [37] |
| sunflower seeds (Natura) | dehulled seeds | total proteins, total carbohydrates, TP | antioxidant capacities | 60–160 °C, 5–120 min, 3 MPa, 1:10–1:30 g/mL | GC-FID, UV-Vis, HPLC | Soxhlet (hexane 67.3%) | [38] |
| cottonseed (Egypt) | cottonseed | linoleic acid, palmatic acid, oleic acid, myristic acid | 180–280 °C, 5–60 min, 1:2–2:1 g/mL | GC-FID, | heating (hexane 89.5%) | [39] | |
| green coffee (Robusta Uganda) | beans | chlorogenic acid | 130–170 °C, 40–90 min, 0–30 % ethanol | HPLC | convention (ethanol 66.7%) | [45] | |
| Nannochloropsis gaditana | fatty acids, omega-3, omega-6, lipid | 156.1–273.9 °C, 6.6–23.4 min, 33–117 g/L | GC-FID, SEM | Soxhlet (n-hexane 100%) | [51] | ||
| Saccharina japonica | gallic, caffeic, vanillic, syringic, chlorogenic, p-hydroxybenzoic acids, etc. | antioxidant (DPPH, ABTS, total antioxidant (FRAP) | 100–250 °C, 5 min, 5 MPa, 0.25–1.00 M ILs | HPLC, UV | convention (DMK 0.2%; DCM 0.3%; Et2O 0.8%; IL 1.6%) | [52] | |
| Haematococcus pluvialis | p-hydroxybenzoic acid, gallic acid, siringic acid, vanillic acid, etc. | antioxidant (ABTS, TEAC), antimicrobial activity | 50–200 °C, 20 min, 10 MPa | HPLC-DAD-MS, SEM, GC-MS | [53] | ||
| Momordica charantia | fruits | TP, gallic acid, gentisic acid, chlorogenic acid | antioxidant (ABTS) | 130–200 °C, 10 MPa, 2–5 mL/min | HPLC, UV | Soxhlet (methanol 4.9%), UAE (methanol 4.0%) | [132] |
| Morus nigra L., Teucrium chamaedrys L., Geranium macrorrhizum L., Symphytum officinale L. | leaves, flowers | TP, chlorogenic acid, gallic acid, vanillic acid, etc. | antioxidant, antifungal, antibacterial, cytotoxic | 60–200 °C, 30 min, 1 MPa, 1:40g/mL | HPLC-DADUV | [133] | |
| Prunus avium L., Prunus cerasus L. | stems | 3 alcohols, 10 organic acids, etc. | antioxidant, antiproliferative | 150 °C, 30 min, 2 MPa | GC-MS, UV | [134] | |
| Castanea sativa | nuts | ellagic acid, feru lic acid, gallic acid, etc. | antioxidant | 120–135 °C, 15–60 min | HPLC | [135] | |
| Solanum tuberosum | potato peel | TP, gallic acid, caffeic acid, chlorogenic acid, protocatechuic acid, etc. | 100–240 °C, 30–120 min, 6 MPa | HPLC, UV | convention (methanol 1.6%; ethanol 2.0%) | [136] | |
| Actinidia deliciosa | pomace | TP, chlorogenic acid, protocatechuic acid, etc. | antioxidant (DPPH, FRAP, ABTS) | 170–225 °C 10–180 min, 5 MPa | UV, HPLC, pH | [137] | |
| hypnea musciformis | chlorogenic, protocatechuic, and gallic acids, TP, TF, etc. | antioxidant (DPPH, ABTS), emulsify | 120–270 °C, 10 min, 1:50–1:150 g/mL | pH, UV, HPLC | [138] | ||
| Carica papaya L. | papaya seeds | TP, 18 phenolic acids, 20 flavonoids, 1 stilbene, etc. | antioxidant (DPPH, β-carotene bleaching) | 70–150 °C, 10 MPa, 1–40 min, 4 mL/min | LC-ESI-MS/MS, UV | Soxhlet (water 37.1%) | [139] |
| Zingiber officinale | ginger rhizome | 12 sugars, 8 diols, 4 phenolic acids, etc. | antimicrobial, cytotoxic | 150 °C, 1 h, 1:10 g/mL | HPLC-ESI-TOFMS | heating (water) | [140] |
| Chlorella sp. microalgae | TP, caffeic acid, ferulic acid, p-coumaric acid | antioxidant (DPPH) | 100–250 °C, 5–20 min | UV, SEM, HPLC | [141] | ||
| Vitis vinifera | vine-canes | TP, flavonoids, phenolic acids, flavonols | antioxidant, antiradical | 125–250 °C, 50 min | HPLC, UV | [142] | |
| Cinnamomum Cassia Blume | cinnamon | coumarin, cinnamic acid, cinnamaldehyde, cinnamyl alcohol, etc. | 110–130°C, 20–60 min, 2–4 MPa, 1:10 g/mL | HPLC | [143] |
| Samples | Medicinal Parts | Compounds Extracted | Extracts Activity | Extraction Conditions | Analytical Methods | Other Extraction Methods (Solvent, Ratios of Yields) | Ref. |
|---|---|---|---|---|---|---|---|
| Phaleria macrocarpa | fruits | mangiferin | 323–423 K, 1–7 h, 0.7–4.0 MPa | HPLC, LC-MS | convention (water 69.6%; ethanol 34.1%; methanol 108%), HRE (water 85.7%; ethanol 60.8%; methanol 115%), Soxhlet (water 86.1%; ethanol 55.8%; methanol 113% methanol) | [36] | |
| Punica granatum L. | pomegranate seed | TP, kaempferol -3-O-rutinoside | antioxidant (DPPH, ABTS) | 80–280 °C, 5–120 min, 1:10–1:50 g/mL, 6.0 MPa | HPLC-DAD, UV, HPLC-ABTS+ | leaching (water 40.6%; methanol 79.7%; ethanol 41.7%; acetone 45.5%), UAE (water11.3%; methanol 20.6%; ethanol 18.9%; acetone 15.2%), Soxhlet (methanol 71.4%; acetone 39.7%) | [144] |
| Teucrium montanum L. | aerial parts | rutin, naringin, epicatechin, etc. | antioxidant (DPPH, FRAP) | 60–200 °C, 30 min, 1–10 MPa, 1:10 g/mL | HPLC-PDA, UV | [145] | |
| Paeonia lactiflora | root | albiflorin, paeoniflorin | 100–260 °C, 10–60 min,10–40 mL/g | HPLC | reflux (water 83.5%), UAE (ethanol 77.8%) | [146] | |
| Morus nigra L. | fruits | TP, TF, cyanidin 3-glucoside, etc. | 40–80 °C, 20–60 min, 2–6 mL/min, 15 MPa | tyrosinase inhibitory activity | UPLC-DAD-ESI-MS/MS | shaker (ethanol:water 116%), UAE (ethanol:water:TFA 134%) | [147] |
| Stevia rebaudiana | leaves | TP, stevioside, rebaudioside A | antioxidants (DPPH) | 100–150°C, 30–60 min, 23 MPa, 1:10 g/mL | HPLC-UV, UV | [148] | |
| Erigeron breviscapus | whole parts | scutellarin, 20 inorganic elements, etc. | antioxidant (DPPH) | 120–140 °C, 5–15 min, 150–420 um | HPLC, HPLC-MS | reflux (methanol 86.1%; ethanol 84.8%) | [149] |
| Mangifera indica L. | leaves | quercetin3-d-glucoside, mangiferin | antioxidant (DPPH) | 100 °C, 4 MPa, 10 g/min, 3 h | UV, HPLC | SCCO2 (20% methanol 18.7%) | [150] |
| Crocus sativus L. | stigmas | picrocrocin, safranal, crocin | 5–15 min, 105–125 °C | GC-MS, UV, HPLC | [151] | ||
| Glycyrrhiza uralensis Fisch | licorice root | TP, glycyrrhetic acid, glycyrrhizin, liquiritin | antioxidant (DPPH, reducing power) | 50–300 °C, 10–60 min, 0.002–5 MPa | HPLC, UV-Vis | [152] |
| Samples | Medicinal Parts | Compounds Extracted | Activity/Mixtures | Extraction Conditions | Analytical Methods | Other Extraction Methods (Solvent, Ratios of Yields) | Ref. |
|---|---|---|---|---|---|---|---|
| Lycium barbarum | berries | total sugar content | antioxidant (FRAP, TEAC), immunomodulatory | 1:30 g/mL, 110 °C, 5 MPa | HPGPC | HWE (water 71.5%), UAE (water 89.9%), UWE (water 132%) | [11] |
| sunflower | sunflower heads | galacturonic acid, pectin | 10–50 min, 2–8 mL/g, 100–140 °C, 0.2–1 MPa | TG/TGA, DSC, UV−vis, FTIR, HPSEC, NMR | [40] | ||
| Aronia melanocarpa | chokeberry stems | 1 amino acid, 8 alcohols, 11 sugars, 2 fatty acids, etc. | antioxidant (DPPH), enzyme inhibitory activity | 130 °C, 3.5 MPa, 20 min, 1:20 g/mL | GC-MS | [49] | |
| Lentinus edodes | fruit bodies | hetero–polysaccharides, xylose, mannose, etc. | antioxidant (OH·, DPPH, ABTS) | 120–160 °C,30–50 min, 0.033–0.05 g/mL | UV-vis, SEM, GC, GPC, FT-IR | [54] | |
| Lentinus edodes | fruit bodies | l-rhamnose, d-arabinose, d-xylose, d-mannose | antioxidant (ABTS), growth inhibitory effect | 100–150 °C, 10–30 min, 5 MPa | FT-IR, UV-Vis, AFM, GC, HP SEC-MALLS | [55] | |
| Lentinus edodes | fruit bodies | polysaccharides, rhamnose, arabinose, xylose, etc. | antioxidant (DPPH, reducing power) | 140 °C, 40 min, 1:25 g/mL, 5 MPa | GC, FT-IR, AFM, SEM | [56] | |
| Lentinula edodes | fruit bodies | TCC, total β-glucan, chitin | HMGCR, immuno- modulatory | 200 °C, 11.7 MPa, 15–60 min | GC-MS, HPSEC, NMR | UAE (water 65.2%), HWE (water 32.3%), SPE (water 33.0%) | [57] |
| Grifola frondosa | fruit bodies | total polysaccharide, total protein | antioxidant (DPPH, reducing power) | 100–230 °C, 2–4 min, 20–100 mesh, 5 MPa | FT-IR, SEM | HWE (water ~87.8%) | [58] |
| Sagittaria sagittifolia L. | fruit bodies | polysaccharides | antioxidant (DPPH, ABTS, reducing power) | 150–190°C, 12–20 min, 1:20–1:40 g/mL, pH 7–9 | FT-IR, 1H and 13C NMR, UV | HWE (water 55.8%) | [59] |
| Sagittaria sagittifolia L. | fruit bodies | l-rhamnose, d-arabinose, d-xylose, d-mannose | antioxidant, immuno-modulatory | 170°C, 16 min | HPLC, GC, SEM, IR, AFM, HPSEC-MALLS | HWE (water 75.6%); UAE (water 96.1%) | [60] |
| Sagittaria sagittifolia L. | fruit bodies | α-pyranose polysaccharide, β-pyranose polysaccharide | immuno-stimulatory | 1 MPa, pH 7,170 °C, 16 min, 30:1 mL/g | IR, GC-FID, UV, HPSEC, AFM | [61] | |
| Cordyceps militaris | fruit bodies | total sugars, protein and uronic acid | 180 °C, 13 min, pH = 8, 21 mL/g | IR, GC, AFM, GPC-MALLS | [62] | ||
| wheat | bran | monosaccharide, etc. | antioxidants (DPPH) | 160–180°C, 5–60 min | HPAEC-PAD, SEC | [153] | |
| Saccharina japonica | fucoidan, fucose, glucose, galactose, mannose, etc. | antioxidant, antimitotic anti-proliferative | 100–180 °C, 5–15 min, 2–8 MPa | FTIR, TGA, UV-Vis | convention (0.05 M HCl 100%) | [154] | |
| Citrus grandis L. | pomelo peel | pectin | 90–120 °C, 3–10 MPa | HPSEC-MALLS | [155] | ||
| Theobroma cacao L. | cacao pod husks | xylose, arabinose, etc. | 121 °C, 30 min, 10.3 MPa | FT-IR, GC-FID, SEM | convention (4% citric acid 76.1%) | [156] | |
| Kappaphycus alvarezii. A | ĸ-carrageenan, glucose, 3,6-anhydrogalactose, etc. | antioxidant (DPPH, ABTS) | 60–180°C, 5 MPa, 5 min | FTIR, TGA, XRD | convention (water 94.3%; water with IL 101%) | [157] | |
| Pseuderanthemum palatiferum | leaves | TCC, monosaccharides | anticoagulant, antioxidant | 150–200°C, 5–10 mL/min | HPLC, GPC, NMR, UV | convention (0.1 M NaOH 48.8%) | [158] |
| wheat | bran | TCC, reducing sugar, arabinose, xylose, etc. | antioxidant, α-amylase inhibitory | 140 °C, 5 MPa, 30 min | SEC-MALLS, FT-IR, DLS, DSC, UV | SBWE (water with citric acid 97.6%); UWE (water with citric acid 103%) | [159] |
| Lycium barbarum L. | fruits | polysaccharides | antioxidant (O2·, OH·, DPPH) | 5 MPa, 25 mL/g, 110 °C, 1 h | UV | HWE (water 86.2%); UAE (water74.9%); UWE (water 111%) | [160] |
| Cocos nucifera L. | defatted coconut | mannose, galactosamine, xylose, rhamnose, etc. | antioxidant, hypoglycaemic, adsorption | 1:10–1:50 g/mL, 10–50 min, 120–200 °C, 20–100 mesh | HPLC, XRD, TGA, DTGA, SEM, FT-IR | [161] | |
| okara | polysaccharides, TP, TF | antioxidant (ABTS, DPPH) | 1:30 g/mL, 160–230 °C, 10 min | UV | [162] | ||
| Saccharina japonica | polysaccharide, fucoidan, alginate | antioxidant (ABTS, DPPH, FRAP) | 100 –150 °C, 1–5 MPa, 1:30–1:50 g/mL | IR, DSC, TGA, 1HNMR, HPLC, HPSEC-ELSD | [163] | ||
| Passiflora edulis | fruit peel | pectic polysaccharide, mannose, glucose, etc. | antioxidant (DPPH) | 100–160 °C, 5.64–7.94 min, 10–30% ethanol | HPLC, UV, viscometer | [164] | |
| Chlorella vulgaris, Sargassum vulgare, Sargassum muticum, Porphyra spp., Cystoseira abies–marina, Undaria pinnatifida and Halopitys incurvus, Rosmarinus officinalis L., Thymus vulgaris, Verbena officinalis | microalgae, algae, leaves | sugar, TP, melanoidins | antioxidant (ABTS, O2¯) | 100–200 °C, 20 min, 10 MPa | UV | [165] | |
| rice bran | bran | protein, TCC, TP | antioxidant (DPPH) | 120–250 °C,0.5–5 mL/min | UV, UV-Vis | [166] | |
| Nizamuddinia zanardinii | TCC, rhamnose, xylose, arabinose, fucoidan, fucose | antioxidant, anticancer, macrophage, etc. | 425 rpm, 10–30 min, 90–150 °C, 0–40 mL/g, 0.75 MPa, 1500 W | FT-IR, GC-MS, SEM, UV, HPSEC-MALLS-RI | [167] | ||
| Dendrobiumnobile Lindl. | stems | polysaccharide, arabinose, galactose, glucose, etc. | antioxidant (OH·, ABTS) | 0.5–1.5 MPa, 5–20 min 120–160 °C, 1:25 g/mL | UV−vis, GPC, HPLC, HPAEC | [168] | |
| Ecklonia maxima | TP, polysaccharide, sulphate, and alginate | antioxidant (ABTS) | 100–180 °C, 5–30 min, 10–50 mL/g, 4 MPa | UV, elemental analysis, ICP-MS | convention (70% ethanol 0%; 0.05 M HCl 20.1%) | [169] | |
| Vitis vinifera | grape pomace | glucose, fructose, galactose, arabinose, mannose, etc. | antimicrobial, antioxidant (DPPH) | 170–210 °C, 10 MPa, 5–10 mL/min | HPLC, UV | [170] | |
| green coffee beans | spent coffee grounds | carbohydrates, phenolics | antioxidant, antibacterial | 150–220 °C, 7 MPa, 10 mL/min, | HPLC, UV, | [171] | |
| Tamarindus indica | seed | TP, xyloglucan | antioxidant (DPPH) | 100–200°C, 1:20 g/mL | SEC, UV | convention (water 74.6%) | [172] |
| Mentha arvensis | leaves | carbohydrates, apocynin | antioxidant (DPPH) | 180–260 °C, 1:20 g/mL, 5 min | HPLC, GC-MS, UV, | [173] |
| Samples | Medicinal Parts | Compounds Extracted | Extraction Conditions | Methods | Other Extraction Methods (Solvent, Ratios of Yields) | Ref. |
|---|---|---|---|---|---|---|
| Essential oils | ||||||
| Thymbra spicata L. | leaves | α-thujene, α-pinene, terpinen-4-ol, p-cymene, γ-terpinene, 1-carvone, thymol, carvacrol, etc. | 100–175 °C, 1–3 mL/min, 2–9 MPa, 30 min | GC-TOF/MS, GC-FID | [12] | |
| Aquilaria malaccensis | leaves | butanal, cyclopentanone, acetoxyacetone, benzaldehyde, acetophenone, creosol, etc. | 100–271 °C, 1–34 min, 0.08–0.22 g/mL | GC-MS, SEM, FT-IR | HD (95.4%) | [22] |
| Mentha piperita L. | peppermint leaves | TP, menthone, menthol, eriocitrin, etc. | 40–160 °C, 10.3 MPa, 1–30 min | GC-MS, FID, HPLC | convention (methanol 53.2%) | [28] |
| Coriandrum sativum L. | coriander seeds | thujene, sabinene, pinene, myrcene, cymene, limonene, ocimene, terpinene, terpinolene, etc. | 100–175 °C, 1–4 mL/min, 0.25–1 mm, 2 MPa, 20 min | GC-FID, GC-MS | HD (1.54-fold), Soxhlet (hexane 1.4-fold) | [174] |
| Coriandrum sativum L. | coriander seeds | 3,4-dimethoxycinnamic acid, coumaric acid, sinapic acid, cis-and trans-linalooloxides, linalool, etc. | 100–200°C, 10–30 min, 3–9 MPa | HPLC-MS/MS, GC-MS | [175] | |
| Kaempferia galangal L. | rhizome | ethyl-p-methoxycinnamate, d-limonene, eucalyptol, tridecane, camphor, borneol, tetradecane, etc. | 120 °C, 10 MPa, 30 min | GC-MS | HD (82.3%), UWE (100%) | [176] |
| Piper betle | leaves | 4-allyl resorcinol, chavibetol | 2 MPa, 10–90 min, 50–250 °C, 0.25–1 mm, 1–4 mL/min | HPLC-UV | convention (water 92.2–111%; methanol 96.6–110%) | [177] |
| Aquilaria malaccensis | leaves | nonacosane, triacontane, pentadecanal, 9-octadecenal, (Z)-, tetradecanal, tetrapentacontane, guaiacol | 100–271 °C, 1–34 min | GC/MS, SEM, BET | [178] | |
| laurel | leaves | α-phellandrene, β-pinene, 1,8-cineole, borneol, nona-3,7-dienol, isobornyl acetate, γ-terpineol, etc. | 15 min, 50–200 °C, 1.5–15 MPa, 0.5–5.0 mL/min | GC-MS, GC-FID | [179] | |
| Citrus hystrix | leaves | linalool, isopulegol, neoisopulegol, citronellal, 4-terpineol, citronellol, geraniol, menthoglycol, etc. | 120–180 °C, 5–20 g/mL, 5–30 min | GC-MS | HD (28.2%) | [180] |
| Coriandrum sativum L. | coriander seeds | α-pinene, β-pinene, camphor, methylchavicol, γ-terpinene, linalool, geraniol, carvacrol, etc. | 100–200 °C, 1:10 g/mL, 2 MPa, 20 min | GC-MS, GC-FID | HD (27.0%), Soxhlet (DCM 6.5-fold), SCCO2 (4-fold) | [181] |
| Lavandula L. | lavender flowers | a-thujene, a-pinene, camphene, sabinene, pinene, myrcene, hexylacetate, terpinene, limonene, etc. | 125 °C, 3 MPa, 30 min | GC-MS, FID | HD (1.2-fold), US-HD (1.3-fold), NaCl-HD (1.3-fold) | [182] |
| Alkaloids | ||||||
| Sophora flavescens Ait. | root | cytisine, matrine, sophoridine, sophocarpine, oxymatrine | 70–190 °C, 5–14 min, 4.0–13.8 MPa | CE | ASE (ethanol 78.1%) | [16] |
| black tea brick | leaves | theophylline, epicatechin gallate, caffeine, etc. | 120–180 °C, 7–42 min, 6–18 mL/min | HPLC | [46] | |
| Symphytum officinale L. | root | lycopsamine, echimidine, lasiocarpine, symviridine | 60–120 °C, 40 min | HPLC, LC-MS, MSn | HRE (methanol 2.8-fold) | [183] |
| hydrastis canadensis | root | hydrastine, berberine | 100–160 °C,1–10 MPa, 5–60 min, 0.5–1.5 mL/min | HPLC-DAD | reflux (methanol 90.8%), UAE (methanol 106%) | [184] |
| cocoa | shells | TP, theobromine, theophylline, caffeine, epicatechin, etc. | 120–220 °C, 15–75 min, 1:10–1:30 g/mL | HPLC, UV | [185] | |
| Musaceae, Beta vulgaris | peels | dopamine, total betacyanin, betaxanthin | 150°C, 5 min, 3 MPa, 1:20 g/mL | HPLC, UV-Vis | infusion (100%), decoction (1.2-fold), maceration (97.4%), UAE (101%), MAE (50.3%) | [186] |
| Coffeea arabica, C. arabica, C. canephora var. robusta, C. canephora var. robusta | coffee silver skin | total sugar, reducing sugar, protein, TP, caffeine, HMF, etc. | 180–270 °C, 10 min, 1.0–5.3 MPa | HPLC, UV | convention (0.1 M HCl 96.6%; 0.1 M NaOH 1.5-fold) | [187] |
| Quinones | ||||||
| Rheum tanguticum | root | damnacanthal | 33–67 min, 100–200°C, 1.4–4.6 mL/min | HPLC, NMR, HSCCC | [17] | |
| Garcinia mangostana Linn | mangosteen pericarps | TP, xanthone | 120–160 °C, 1–10 MPa, 5–60 min, 10–30% DES | UV-vis, FT-IR, SEM | [188] | |
| Phaleria macrocarpa | mahkota dewa fruits | mangiferin | 4.0 MPa, 5 h, 50–150 °C | HPLC | [189] | |
| Lithospermum erythrorhizon | root | shikonin, acetylshikonin, β-dimethylacrylshikonin, etc. | 40–60 mesh, 120 °C, 5 MPa | UV, HPLC-ELSD | SCCO2 (86.3%), Soxhlet (ethyl acetate 95.4%), UWE (1.4-fold) | [190] |
| Morinda citrifolia | root | alizarin | 4 MPa, 150 and 220 °C, 1.6–4 mL/min | RP-HPLC-UV | [191] | |
| Morinda citrifolia | root | 1,2-dihydroxyanthraquinone, alizarin | 110–220 °C, 2–6 mL/min | UV-Vis | ethanol (3 d) | [192] |
| Morinda citrifolia | root | 4 MPa, 150–200 °C, 2–6 mL/min | UV-Vis | convention (ethanol 81.16%), Soxhlet (ethanol 97.94%), UAE (ethanol 79.62%) SWBE (96.41%) | [193] | |
| Terpenes | ||||||
| Hedyotis diffusa Willd. | whole plants | ursolic acid | 120–200 °C, 10–50 min, 20–40 mL/g, 0.6–3.0 MPa | HPLC-ESI-TOF-MS | maceration (ethanol 58.8%), HRE (ethanol 78.4%), UAE (ethanol 90.4%), MAE (ethanol 74.9%) | [13] |
| Centella asiatica | whole plants | asiatic acid, asiaticoside | 100–250°C, 10–40 MPa, 5 h | HPLC, DLS | [14] | |
| basil, oregano | leaves | limonene, citronellol, etc. | 100 and 150 °C, 10 min | GC-FID | [48] | |
| Ganoderma lucidum | fruits | ganodermanon-triol, ganoderic acids, lucidumol | 100–200 °C, 5–10 MPa, 5–60 min | HPLC, SEC-UV, SEM, MALDI-TOF | [194] | |
| Orostachys japonicus | stems, leaves | triterpene, camellia, etc. | 110–260 °C, 5–20 min, 10 MPa | HPLC-MS | [195] | |
| Betula pendula | birch bark | betulinic acid | 160–200 °C, 10–30min, 10 MPa | HPLC | [196] | |
| Inula racemose | plants | igalan, soalantolactone, alantolactone | 23.2–56.8 min, 1.3–4.7 mL/min, 129.5–230.5 °C | HPLC, 1H-NMR 13C-NMR, MS | Soxhlet (ethanol 100%), UAE (ethanol 70.36%), SCCO2 (76.06%) | [197] |
| Semen richonsanthis | seeds | 3,29-dibenzoylkarounidiol, polysaccharides | 80–160 °C, 5.0–30.0 min | HPLC, UV, SEM | [198] | |
| Cucurbita pepo | pumpkin peel | 14 carotenoid compounds | 120 °C, 3 h, 5 MPa | UV, HPLC | SCCO2 (75.4%) | [199] |
| Betula pendula | birch bark | sesquiterpenes, steroids | 10 min, 100–200 °C | LC, GC/MS, NMR | [200] | |
| S. rebaudiana | Bertoni leaves | steviol glycosides, tannins, chlorophyll A | 100–160 °C, 5–10 min, 10.34 MPa, 1:3 g/mL | HPLC, UV, UV/Vis | [201] | |
| Lignans | ||||||
| Linum usitatissimum L. | flaxseed | SDG lignan, phenolics, flavonoids | 160–180 °C, 5–60 min, 10 MPa | HPLC-MS/MS, UV | [41] | |
| Sesamum indicum L. | sesame seeds | lignans, TP, flavonoids, flavonols | 140–220 °C, 8–14 MPa, 0–95% ethanol, 0–75 min | UV | [42] | |
| Linum usitatissimum L. | flaxseed | total fat content, SDG lignan | 120–180 °C, 15–90 min, 10–13.8 MPa | HPLC-MS/MS, UV | [43] | |
| Sinopodophyllum hexandrum | root | podophyllotoxin | 12 mL/g, 3 MPa, 2ml/min, 120–240 °C | HPLC | [202] | |
| Steroids | ||||||
| Pfaffia glomerata, Amaranthaceae | ginseng root | sugar, fructooligosaccharides, beta-ecdysone | 80–180 °C, 5–15 min, 2–12 MPa | HPLC-ELSD, HPLC | [24] | |
| Panax ginseng C.A. Meyer | ginseng root | TP, maltol, panaxadiol, panaxatriol | 150–200 °C, 5–30 min, 100 MPa | HPLC, UV | convention (water 32.6%; methanol 24.1%; ethanol 18.7%) | [25] |
| Panax ginseng C.A. Meyer | ginseng root | total ginsenosides, total sugar, 1-oleanane ginsenosides, etc. | 120–200 °C, 20 min, 1:20 g/mL, 6.0 MPa | FT-IR, UV, UFLC-MS/MS | heating (water, 30.9%; ethanol 94.4%) | [26] |
| grapevine | root, wood, cane | E-piceid, E-piceatannol, E-resveratrol, E-parthenocissin, etc. | 100–190 °C, 5–30 min, 10 MPa | LC-DAD/ESI-IT, Q-TOF, NMR | ASE (116% for cane; 103% for wood; 1.5-fold for root) | [203] |
| Withania somnifera L. | root leaves | TP, withanoside IV V, withaferin A, withanolide A, B | 100–200 °C, 10–30 min, 10 MPa | HPLC, UV | maceration (water 31.7%), Soxhlet (ethanol 39.2%), MAE (methanol 45.8%) | [204] |
| Acanthophyllum glandulosum | root | saponin | 121 °C, 0.15MPa, 15 min, pH 4–9 | FT-IR, UV-vis, HPLC | [205] | |
| Vaccaria segetalis | cowcock seed | vaccarosides, segetosides | 125–175 °C, 15–180 min | USE (methanol 46.8%; water 27.9%; ethanol 5.2%) | [206] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. https://doi.org/10.3390/molecules26134004
Cheng Y, Xue F, Yu S, Du S, Yang Y. Subcritical Water Extraction of Natural Products. Molecules. 2021; 26(13):4004. https://doi.org/10.3390/molecules26134004
Chicago/Turabian StyleCheng, Yan, Fumin Xue, Shuai Yu, Shichao Du, and Yu Yang. 2021. "Subcritical Water Extraction of Natural Products" Molecules 26, no. 13: 4004. https://doi.org/10.3390/molecules26134004
APA StyleCheng, Y., Xue, F., Yu, S., Du, S., & Yang, Y. (2021). Subcritical Water Extraction of Natural Products. Molecules, 26(13), 4004. https://doi.org/10.3390/molecules26134004