New Precursors to 3-Sulfanylhexan-1-ol? Investigating the Keto–Enol Tautomerism of 3-S-Glutathionylhexanal
Abstract
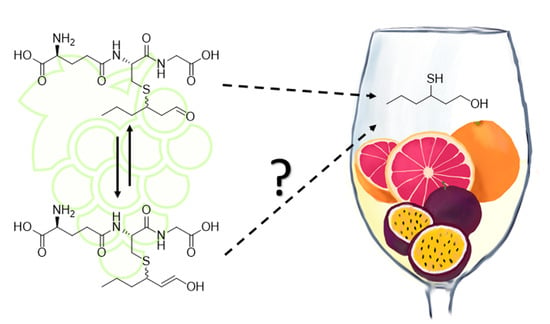
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Analysis of Spectra
3.3. Synthesis of 3-S-Glutathionylhexanal, Glut-3SH-al
3.4. Purification of Synthesised Compounds
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of new volatile thiols in the aroma of Vitis vinifera L. var. Sauvignon blanc wines. Flavour Fragr. J. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Lund, C.M.; Thompson, M.K.; Benkwitz, F.; Wohler, M.W.; Triggs, C.M.; Gardner, R.; Heymann, H.; Nicolau, L. New Zealand Sauvignon blanc distinct flavor characteristics: Sensory, chemical, and consumer aspects. Am. J. Enol. Vitic. 2009, 60, 1–12. [Google Scholar]
- Benkwitz, F.; Tominaga, T.; Kilmartin, P.A.; Lund, C. Identifying the chemical composition related to the distinct aroma characteristics of New Zealand Sauvignon blanc wines. Am. J. Enol. Vitic. 2011, 63, 62–72. [Google Scholar] [CrossRef]
- Darriet, P.; Tominaga, T.; Lavigne, V.; Boidron, J.-N.; Dubourdieu, D. Identification of a powerful aromatic component of Vitis vinifera L. var. Sauvignon wines: 4-mercapto-4-methylpentan-2-one. Flavour Fragr. J. 1995, 10, 385–392. [Google Scholar] [CrossRef]
- Chen, L.; Capone, D.L.; Jeffery, D.W. Analysis of Potent Odour-Active Volatile Thiols in Foods and Beverages with a Focus on Wine. Molecules 2019, 24, 2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mafata, M.; Stander, M.A.; Thomachot, B.; Buica, A. Measuring Thiols in Single Cultivar South African Red Wines Using 4,4-Dithiodipyridine (DTDP) Derivatization and Ultraperformance Convergence Chromatography-Tandem Mass Spectrometry. Foods 2018, 7, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musumeci, L.E.; Ryona, I.; Pan, B.S.; Loscos, N.; Feng, H.; Cleary, M.T.; Sacks, G.L. Quantification of Polyfunctional Thiols in Wine by HS-SPME-GC-MS Following Extractive Alkylation. Molecules 2015, 20, 12280–12299. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.C.; Deed, R.C. Chemical Reaction of Glutathione and trans-2-Hexenal in Grape Juice Media to Form Wine Aroma Precursors: The Impact of pH, Temperature, and Sulfur Dioxide. J. Agric. Food Chem. 2018, 66, 1214–1221. [Google Scholar] [CrossRef]
- Bonnaffoux, H.; Delpech, S.; Rémond, E.; Schneider, R.; Roland, A.; Cavelier, F. Revisiting the evaluation strategy of varietal thiol biogenesis. Food Chem. 2018, 268, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, D.W. Spotlight on Varietal Thiols and Precursors in Grapes and Wines. Aust. J. Chem. 2017, 69, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef] [PubMed]
- Thibon, C.; Böcker, C.; Shinkaruk, S.; Moine, V.; Darriet, P.; Dubourdieu, D. Identification of S-3-(hexanal)-glutathione and its bisulfite adduct in grape juice from Vitis vinifera L. cv. Sauvignon blanc as new potential precursors of 3SH. Food Chem. 2016, 199, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Jelley, R.E.; Duhamel, N.; Barker, D.; Fedrizzi, B. A convenient synthesis of amino acid-derived precursors to the important wine aroma 3-sulfanylhexan-1-ol (3SH). Tetrahedron Lett. 2020, 61, 151663. [Google Scholar] [CrossRef]
- Muhl, J.R.; Pilkington, L.I.; Deed, R.C. First synthesis of 3-S-glutathionylhexanal-d8 and its bisulfite adduct. Tetrahedron Lett. 2020, 61, 152100. [Google Scholar] [CrossRef]
- Nichols, M.A.; Waner, M.J. Kinetic and mechanistic studies of the deuterium exchange in classical keto-enol tautomeric equilibrium reactions. J. Chem. Educ. 2010, 87, 952–955. [Google Scholar] [CrossRef]
- Guthrie, J.P. The enol content of simple carbonyl compounds: An approach based upon p K a estimation. Can. J. Chem. 1979, 57, 1177–1185. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhl, J.R.; Pilkington, L.I.; Deed, R.C. New Precursors to 3-Sulfanylhexan-1-ol? Investigating the Keto–Enol Tautomerism of 3-S-Glutathionylhexanal. Molecules 2021, 26, 4261. https://doi.org/10.3390/molecules26144261
Muhl JR, Pilkington LI, Deed RC. New Precursors to 3-Sulfanylhexan-1-ol? Investigating the Keto–Enol Tautomerism of 3-S-Glutathionylhexanal. Molecules. 2021; 26(14):4261. https://doi.org/10.3390/molecules26144261
Chicago/Turabian StyleMuhl, Jennifer R., Lisa I. Pilkington, and Rebecca C. Deed. 2021. "New Precursors to 3-Sulfanylhexan-1-ol? Investigating the Keto–Enol Tautomerism of 3-S-Glutathionylhexanal" Molecules 26, no. 14: 4261. https://doi.org/10.3390/molecules26144261
APA StyleMuhl, J. R., Pilkington, L. I., & Deed, R. C. (2021). New Precursors to 3-Sulfanylhexan-1-ol? Investigating the Keto–Enol Tautomerism of 3-S-Glutathionylhexanal. Molecules, 26(14), 4261. https://doi.org/10.3390/molecules26144261