Morphologically Diverse Micro- and Macrostructures Created via Solvent Evaporation-Induced Assembly of Fluorescent Spherical Particles in the Presence of Polyethylene Glycol Derivatives
Abstract
:1. Introduction
2. Results and Discussion
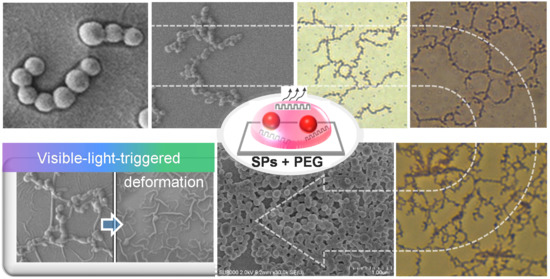
2.1. Growth of Organic SP Building Blocks into Diverse Micro- and Macrostructures
2.2. Control Experiments: Need of PEG Chains for Morphological Growth
2.3. In Situ Morphological Evolution Processes at Higher PEG Fractions
2.4. UV-Vis Absorption and IR Measurements
2.5. Light-Sensitive Interconnected Structures
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. PEG-Mediated SP Assembly into Various Microstructures
4.3. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kato, T.; Sagara, Y. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar]
- Kim, H.; Kim, T.; Lee, M. Responsive nanostructures from aqueous assembly of rigid-flexible block molecules. Acc. Chem. Res. 2011, 44, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Song, P.; Tong, A. Reversible thermochromism of aggregation-induced emission-active benzophenone azine based on polymorph-dependent excited-state intramolecular proton transfer fluorescence. J. Phys. Chem. C 2013, 117, 3467–3474. [Google Scholar] [CrossRef]
- Gopikrishna, P.; Meher, N.; Iyer, P.K. Functional 1,8-naphthalimide AIE/AIEEgens: Recent advances and prospects. ACS Appl. Mater. Interfaces 2017, 10, 12081–12111. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Moran, M.J.; Aprahamian, I. New molecular switch architectures. Proc. Natl. Acad. Sci. USA 2018, 115, 9414–9422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, H.; Liu, B.; Liu, J.; Wei, P.; Zhang, H.; Han, T.; Qi, J.; Lam, J.W.Y.; Zhang, W.; Tang, B.Z. “Seeing” and Controlling Photoisomerization by (Z)-/(E)-Isomers with Aggregation-Induced Emission Characteristics. ACS Nano 2019, 13, 12120–12126. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, X.; Wang, Y.; Li, Z. Supramolecular self-assembly of perylene bisimide derivatives assisted by various groups. Langmuir 2019, 35, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Aggarwal, H.; Srivastava, A. Of Twists and Curves: Electronics, Photophysics, and Upcoming Applications of Non-Planar Conjugated Organic Molecules. Chem. Eur. J. 2020, 26, 10653–10675. [Google Scholar] [CrossRef]
- Sun, C.; Jiang., X.; Li., B.; Kong, X.Z. Fluorescence behavior and mechanisms of poly(ethylene glycol) and their applications in Fe3+ and Cr6+ detections, data encryption, and cell imaging. ACS Sustain. Chem. Eng. 2021, 9, 5166–5178. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Fu, H.; Peng, A.; Ma, Y.; Liao, Q.; Yao, J. Construction and optoelectronic properties of organic one-dimensional nanostructures. Acc. Chem. Res. 2010, 43, 409–418. [Google Scholar] [CrossRef]
- Shen, B.; Kim, Y.; Lee, M. Supramolecular chiral 2D materials and emerging functions. Adv. Mater. 2020, 32, 1905669. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Chen, Y.; Yang, W.; Yao, J.; Zhu, L.; Shuai, Z. Low-Dimensional aggregates from stilbazolium-like dyes. Angew. Chem. Int. Ed. 2004, 43, 4060–4063. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.C.; Stupp, S.I. Molecular self-assembly into one-dimensional nanostructures. Acc. Chem. Res. 2008, 41, 1674–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Rooy, S.L.; El-Zahab, B.; Li, M.; Das, S.; Broering, E.; Chandler, L.; Warner, I.M. Fluorescent one-dimensional nanostructures from a group of uniform materials based on organic salts. Chem. Commun. 2011, 47, 8916. [Google Scholar] [CrossRef]
- Cantekin, S.; de Greef, T.F.A.; Palmans, A.R.A. Benzene-1,3,5-tricarboxamide: A versatile ordering moiety for supramolecular chemistry. Chem. Soc. Rev. 2012, 41, 6125–6137. [Google Scholar] [CrossRef]
- Kim, Y.; Li, W.; Shin, S.; Lee, M. Development of Toroidal Nanostructures by Self-Assembly: Rational Designs and Applications. Acc. Chem. Res. 2013, 46, 2888–2897. [Google Scholar] [CrossRef]
- Hernández-Toledo, H.; Torrens, H.; Flores-Álamo, M.; De Cola, L.; Moreno-Alcántar, G. Self-Assembly and Aggregation-Induced Emission in Aqueous Media of Responsive Luminescent Copper(I) Coordination Polymer Nanoparticles. Chem. Eur. J. 2021, 27, 8308–8314. [Google Scholar] [CrossRef]
- Maheshwari, V.; Kane, J.; Saraf, R. Self-Assembly of a Micrometers-Long One-Dimensional network of cemented Au Nanoparticles. Adv. Mater. 2008, 20, 284–287. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.A.; Campolongo, M.J.; Tan, S.J.; Luo, D.; Cheng, W.; Cha, J.J.; Umbach, C.C. Free-Standing Nanoparticle Superlattice Sheets controlled by DNA. Nat. Mater. 2009, 8, 519–525. [Google Scholar]
- Becker, L.; De Cola, A. Studer, Assembly of linear chains consisting of alternating silica beads and zeolite L crystals by nitroxide exchange reactions. J. Mater. Chem. C 2013, 1, 3287–3290. [Google Scholar] [CrossRef]
- Mahmoud, M.A. Dynamic template for assembling nanoparticles into highly ordered Two-Dimensional arrays of different structures. J. Phys. Chem. C 2015, 119, 305–314. [Google Scholar] [CrossRef]
- Dong, D.; Fu, R.; Shi, Q.; Cheng, W. Self-assembly and characterization of 2D Plasmene Nanosheets. Nat. Protoc. 2019, 14, 2691–2706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, D.; Möhwald, H. Fabrication of Multiplex Quasi-Three-Dimensional grids of One-Dimensional nanostructures via stepwise colloidal lithography. Nano Lett. 2007, 7, 3410–3413. [Google Scholar] [CrossRef] [PubMed]
- Osberg, K.D.; Schmucker, A.L.; Senesi, A.J.; Mirkin, C.A. One-Dimensional Nanorod Arrays: Independent control of composition, length, and interparticle spacing with nanometer precision. Nano Lett. 2011, 11, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Henrist, C.; Cloots, R. Nanosphere lithography: A powerful method for the controlled manufacturing of nanomaterials. J. Nanometer. 2013, 2013, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kalia, S.; Haldorai, Y. (Eds.) Organic-Inorganic Hybrid Nanomaterials, Frontiers in Nanofabrication via Self-Assembly of Hybrid Materials into Low Dimensional Nanostructures; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Li, D.; Xia, Y. Fabrication of titania nanofibers by electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, S. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 43, 4423–4448. [Google Scholar] [CrossRef]
- Tao, A.; Yang, P.; Sinsermsuksakul, P. Tunable plasmonic lattices of Silver Nanocrystals. Nat. Nanotechnol. 2007, 2, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrovic, V.; Greshnykh, D.; Randjelovic, I.; Frömsdorf, A.; Kornowski, A.; Roth, S.V.; Klinke, C.; Weller, H. Preparation and electrical properties of Cobalt−Platinum nanoparticle monolayers deposited by the Langmuir−Blodgett technique. ACS Nano 2009, 2, 1123–1130. [Google Scholar] [CrossRef] [Green Version]
- Edler, K.J.; Yang, B. Formation of mesostructured thin films at the air-liquid interface. Chem. Soc. Rev. 2013, 42, 3765–3776. [Google Scholar] [CrossRef]
- Roger, K.; Sparr, E.; Wennerström, H. Evaporation, Diffusion and self-assembly at drying interfaces. Phys. Chem. Chem. Phys. 2018, 20, 10430–10438. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Lay, C.L.; Shi, W.; Lee, H.K.; Yang, Y.; Li, S.; Lin, X.Y. Creating two self-assembly micro-environments to achieve supercrystals with dual structures using polyhedral nanoparticles. Nat. Commun. 2018, 9, 2769. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Mayers, B.; Xia, Y. Transformation of Silver Nanospheres into Nanobelts and Triangular Nanoplates through a Thermal Process. Nano Lett. 2003, 3, 675–679. [Google Scholar] [CrossRef]
- Fukao, M.; Sugawara, A.; Shimojima, A.; Fan, W.; Arunagirinathan, M.A.; Tsapatsis, M.; Okubo, T. One-Dimensional assembly of silica nanospheres mediated by block copolymer in liquid phase. J. Am. Chem. Soc. 2009, 131, 16344–16345. [Google Scholar] [CrossRef]
- Zhou, S.; Sakamoto, T.; Wang, J.; Sugawara. One-Dimensional Assembly of Silica Nanospheres: Effects of Nonionic Block Copolymers. Langmuir 2012, 28, 13181–13188. [Google Scholar] [CrossRef]
- Kim, P.Y.; Oh, J.; Nam, J. Controlled co-assembly of nanoparticles and polymer into ultralong and continuous one-dimensional nanochains. J. Am. Chem. Soc. 2015, 137, 8030–8033. [Google Scholar] [CrossRef]
- Lee, M.; Lim, S.; Ha, J.; Choi, S. Green Synthesis of High-Purity Mesoporous Gold Sponges Using Self-Assembly of Gold Nanoparticles Induced by Thiolated Poly(ethylene gly-col). Langmuir 2016, 32, 5937–5945. [Google Scholar] [CrossRef] [PubMed]
- Vaknin, D.; Wang, W.; Islam, F.; Zhang, H. Polyethylene-Glycol-Mediated Self-Assembly of Magnetite Nanoparticles at the Liquid/Vapor Interface. Adv. Mater. Interfaces 2018, 5, 1701149. [Google Scholar] [CrossRef] [Green Version]
- Nayak, S.; Fieg, M.; Wang, W.; Bu, W.; Mallapragada, S.; Vaknin, D. Effect of (poly) electrolytes on the interfacial assembly of poly(ethylene glycol)-functionalized gold nanoparticles. Langmuir 2019, 35, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- He, X.H.; Ji, Y.; Xie, J.; Hu, W.; Jia, K.; Liu, X. Emulsion solvent evaporation induced self-assembly of polyarylene ether nitrile block copolymers into functional metal coordination polymeric microspheres. Polymer 2020, 186, 122024. [Google Scholar] [CrossRef]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzán, L.M. Directed Self-Assembly of nanoparticles. ACS. Nano. 2010, 4, 3591–3605. [Google Scholar] [CrossRef]
- Zhou, J.; Ke, F.; Tong, Y.; Li, Z.; Liang, D. Solution behavior of copolymers with poly (ethylene oxide) as the “hydrophobic” block. Soft Matter 2011, 7, 9956. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Mallapragada, S.; Travesset, A.; Vaknin, D. Macroscopic and tunable nanoparticle superlattices. Nanoscale 2017, 9, 164–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [Green Version]
- Rahme, K.; Chen, L.; Hobbs, R.G.; Morris, M.A.; O’Driscolle, C.; Holmes, J.D. PEGylated gold nanoparticles: Polymer quantification as a function of PEG lengths and nanoparticle dimensions. RSC Adv. 2013, 3, 6085–6094. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Mounsamy, M.; Lauth-de Viguerie, N.; Coppel, Y.; Harrisson, S.; Destarac, M.; Mingotaud, C.; Kahn, M.L.; Marty, J.-D. Luminescent zinc oxide nanoparticles: From stabilization to slow digestion depending on the nature of polymer coating. Polymer Chem. 2019, 10, 145–154. [Google Scholar] [CrossRef]
- Pranjali, P.; Meher, M.K.; Raj, R.; Prasad, N.; Poluri, K.M.; Kumar, D.; Guleria, A. Physicochemical and Antibacterial Properties of PEGylated Zinc Oxide Nanoparticles Dispersed in Peritoneal Dialysis Fluid. ACS Omega 2019, 4, 19255–19264. [Google Scholar]
- Zhao, L.; Yang, W.; Ma, Y.; Yao, J.; Li, Y.; Liu, H. Perylene nanotubes fabricated by the template method. Chem. Commun. 2003, 2442–2443. [Google Scholar] [CrossRef]
- Fu, H.; Xiao, D.; Yao, J.; Yang, G. Nanofibers of 1,3-Diphenyl-2-pyrazoline induced by cetyltrimethylammonium bromide micelles. Angew. Chem. Int. Ed. 2003, 42, 2883–2886. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guo, Y.G.; Liang, H.; Wan, L.; Jiang, L. Three-Dimensional Self-Organization of supramolecular self-assembled porphyrin hollow hexagonal nanoprisms. J. Am. Chem. Soc. 2005, 127, 17090–17095. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- An, B.; Kwon, S.; Jung, S.; Park, S.Y. Enhanced emission and its switching in fluorescent organic nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, B.; Gierschner, J.; Park, S.Y. π-Conjugated cyanostilbene derivatives: A unique self-assembly motif for molecular nanostructures with enhanced emission and transport. Acc. Chem. Res. 2012, 45, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced emission: New vistas at the aggregate level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Han, M.; Cho, S.J.; Norikane, Y.; Shimizu, M.; Seki, T. Assembly of an achiral chromophore into light-responsive helical nanostructures in the absence of chiral components. Chem. Eur. J. 2016, 22, 3971–3975. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Hara, M.; Seki, T.; Cho, S.J.; Shimizu, M.; Matsuura, K.; Cheong, H.; Kim, J.Y.; Oh, J.; Jung, J.; et al. A trigonal molecular assembly system with the dual light-driven functions of phase transition and fluorescence switching. J. Mater. Chem. C 2019, 7, 2276–2282. [Google Scholar] [CrossRef]
- Israelachvili, J. The Different faces of Poly(Ethylene Glycol). Proc. Natl. Acad. Sci. USA 1997, 94, 8378–8379. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Chao, Z.; Weifeng, L.; Rundong, H.; Qiuming, W.; Hong, C.; Lingyan, L.; Shengfu, C.; Jie, Z. Binding Characteristics between Polyethylene Glycol (PEG) and proteins in aqueous solution. J. Mater. Chem. B 2014, 2, 2983–2992. [Google Scholar]
- Cao, Z.; Jiang, S. Super-hydrophilic zwitterionic poly(carboxybetaine) and Amphiphilic Non-Ionic Poly(ethylene glycol) for stealth nanoparticles. Nano Today 2012, 7, 404–413. [Google Scholar] [CrossRef]
- Özdemir, C.; Güner, A. solubility profiles of poly(ethylene glycol)/solvent systems, I: Qualitative comparison of solubility parameter approaches. Eur. Polym. J. 2007, 43, 3068–3093. [Google Scholar] [CrossRef]
- Diönç, C.Ö.; Kiübarer, G.; Güner, A. Solubility profiles of poly(ethylene glycol)/solvent systems. II. Comparison of thermodynamic parameters from viscosity measurements. J. Appl. Polym. Sci. 2010, 117, 1100–1119. [Google Scholar] [CrossRef]
- Kato, T.; Nakamura, K.; Kawaguchi, M.; Takahashi, A. Quasielastic light scattering measurements of polystyrene latices and conformation of poly(oxyethylene) adsorbed on the latices. Polym. J. 1981, 13, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, S.; Imai, G.; Suzuki, J.; Miyahara, A.; Kitano, T.; Ito, K. aqueous solution properties of oligo- and poly(ethylene oxide) by static light scattering and intrinsic viscosity. Polymer 1997, 38, 2885–2891. [Google Scholar] [CrossRef]
- Park, J.; Fong, P.M.; Lu, J.; Russell, K.S.; Booth, C.J.; Saltzman, W.M.; Fahmy, T.M. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomedicine 2009, 5, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.; Liu, P.; Liang, X. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.H.J.; Zuckerman, J.E.; Webster, P.; Davis, M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar] [CrossRef] [Green Version]
- Zalipsky, S. Functionalized poly(ethylene glycols) for preparation of biologically relevant conjugates. Bioconjug. Chem. 1995, 6, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Ichimura, K. In-Plane and Tilt Reorientation of p-Methoxyazobenzene side chains tethered to liquid crystalline polymethacrylates by irradiation with 365 nm light. Macromolecules 2001, 34, 90–98. [Google Scholar] [CrossRef]
- Kwon, J.E.; Park, S.; Park, S.Y. Realizing molecular pixel system for full-color fluorescence reproduction: RGB-emitting molecular mixture free from energy transfer crosstalk. J. Am. Chem. Soc. 2013, 135, 11239–11246. [Google Scholar] [CrossRef] [PubMed]
- Gabor, G.; Frei, Y.; Gegiou, D.; Kaganowitch, M.; Fischer, E. Tautomerism and geometric isomerism in arylazo-phenols and naphthols. part III. Orthohydroxy derivatives and their reversible photochemical reactions. Isr. J. Chem. 1967, 5, 193–211. [Google Scholar] [CrossRef]
- Douhal, A.; Sanz, M.; Tormo, L. Femtochemistry of orange II in solution and in chemical and biological nanocavities. Proc. Natl. Acad. Sci. USA 2005, 102, 18807–18812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, G.; Guan, P.-J.; Fang, W.-H. Photoinduced proton transfer and isomerization in a hydrogen-bonded aromatic azo compound: A CASPT2//CASSCF study. J. Phys. Chem. A 2014, 118, 4732–4739. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Cho, S.J.; Norikane, Y.; Shimizu, M.; Kimura, A.; Tamagawa, T.; Seki, T. Multistimuli-responsive azobenzene nanofibers with aggregation-induced emission enhancement characteristics. Chem. Commun. 2014, 50, 15815–15818. [Google Scholar] [CrossRef]
- Han, M.; Takeoka, Y.; Seki, T. Facile morphological control of fluorescent nano/microstructures via self-assembly and phase separation of trigonal azobenzenes showing aggregation-induced emission enhancement in polymer matrices. J. Mater. Chem. C 2015, 3, 4093–4098. [Google Scholar] [CrossRef]
- Rauf, M.A.; Hisaindee, S.; Saleh, N. Spectroscopic studies of keto-enol tautomeric equilibrium of azo dyes. RSC. Adv. 2015, 5, 18097–18110. [Google Scholar] [CrossRef]
- The melting points of mPEG1000 and PEG2000 are 36−44 °C and 51−55 °C, Respectively. Available online: https://www.tcichemicals.com/JP/ja/p/P2034 (accessed on 14 July 2021).
- Draper, E.R.; Adams, D.J. How should multicomponent supramolecular gels be characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Abe, I.; Matsuura, K.; Takeoka, Y.; Seki, T. Morphologically Diverse Micro- and Macrostructures Created via Solvent Evaporation-Induced Assembly of Fluorescent Spherical Particles in the Presence of Polyethylene Glycol Derivatives. Molecules 2021, 26, 4294. https://doi.org/10.3390/molecules26144294
Han M, Abe I, Matsuura K, Takeoka Y, Seki T. Morphologically Diverse Micro- and Macrostructures Created via Solvent Evaporation-Induced Assembly of Fluorescent Spherical Particles in the Presence of Polyethylene Glycol Derivatives. Molecules. 2021; 26(14):4294. https://doi.org/10.3390/molecules26144294
Chicago/Turabian StyleHan, Mina, Ikue Abe, Kazunori Matsuura, Yukikazu Takeoka, and Takahiro Seki. 2021. "Morphologically Diverse Micro- and Macrostructures Created via Solvent Evaporation-Induced Assembly of Fluorescent Spherical Particles in the Presence of Polyethylene Glycol Derivatives" Molecules 26, no. 14: 4294. https://doi.org/10.3390/molecules26144294
APA StyleHan, M., Abe, I., Matsuura, K., Takeoka, Y., & Seki, T. (2021). Morphologically Diverse Micro- and Macrostructures Created via Solvent Evaporation-Induced Assembly of Fluorescent Spherical Particles in the Presence of Polyethylene Glycol Derivatives. Molecules, 26(14), 4294. https://doi.org/10.3390/molecules26144294