Polymeric Microfiltration Membranes Modification by Supercritical Solvent Impregnation—Potential Application in Open Surgical Wound Ventilation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Supercritical Solvent Impregnation (SSI)
2.2.2. FTIR Analyses
2.2.3. Microscopy
2.2.4. Tests in a Cross-Filtration Unit
2.2.5. Release of Carvacrol in Carbon Dioxide
2.2.6. Thoracic Cavity Model Protocol
2.2.7. Microbial Analyses
3. Results and Discussion
3.1. Supercritical Solvent Impregnation
3.2. FTIR Analyses
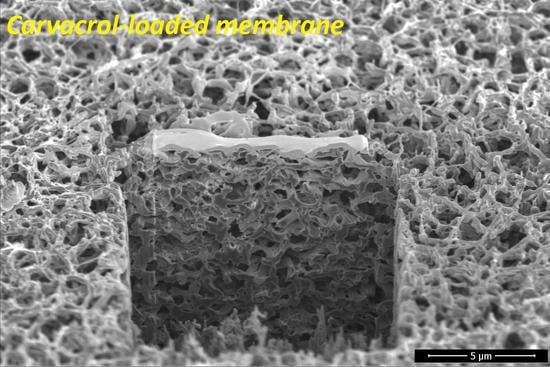
3.3. Modification Impact on Membrane Microstructure
3.4. Carvacrol Release in Carbon Dioxide
3.5. Thoracic Cavity Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A










References
- Weidner, E. Impregnation via supercritical CO2—What we know and what we need to know. J. Supercrit. Fluids 2018, 134, 220–227. [Google Scholar] [CrossRef]
- Kiellow, A.W.; Henriksen, O. Supercritical wood impregnation. J. Supercrit. Fluids 2009, 50, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Iversen, S.B.; Larsen, T.; Henriksen, O.; Felsvang, K. The world’s first commercial supercritical wood treatement plant. In The International Society for the Advancement of Supercritical Fluids, Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28−30 April 2003; Institute National Polytechnique de Lorraine: Vandoeuvre-lès-Nancy, France, 2003. [Google Scholar]
- Van der Kraan, M.; Cid, M.V.F.; Woerlee, G.F.; Veugelers, W.J.T.; Witkamp, G.J. Dyeing of natural and synthetic textiles in supercritical carbon dioxide with disperse reactive dyes. J. Supercrit. Fluids 2007, 40, 470–476. [Google Scholar] [CrossRef]
- Dyecoo. Available online: http://www.dyecoo.com/ (accessed on 20 May 2021).
- Wolf, C.; Maninger, J.; Lederer, K.; Fruhwirth-Smounig, H.; Gamse, T.; Marr, R. Stabilisation of crosslinked ultra-high molecular weight polyethylene (UHMW-PE)-acetabular components with α-tocopherol. J. Mater. Sci. Mater. Med. 2006, 17, 1323–1331. [Google Scholar] [CrossRef]
- Thomas, G.; Rolf, M.; Christian, W.; Klaus, L. Supercritical CO2 impregnation of polyethylene components for medical purposes. Hem. Ind. 2007, 61, 229–232. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.; Guerra, J.P.; Duarte, A.R.; Duarte, C.M.; Leite, E.O.; Gil, M.H.; de Sousa, H.C. Development of therapeutic contact lenses using a supercritical solvent impregnation method. J. Supercrit. Fluids 2010, 52, 306–316. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.; Duarte, C.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Gil, M.H.; de Sousa, H.C. Anti-glaucoma drug-loaded contact lenses prepared using supercritical solvent impregnation. J. Supercrit. Fluids 2010, 53, 165–173. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Galotto, M.J.; Guarda, A.; Romero, J. Supercritical impregnation for food applications: A review of the effect of the operational variables on the active compound loading. Crit. Rev. Food Sci. 2020, 60, 1290–1301. [Google Scholar] [CrossRef]
- Zizovic, I.; Trusek, A.; Tyrka, M.; Moric, I.; Senerovic, I. Functionalization of polyamide microfiltration membranes by supercritical solvent impregnation. J. Supercrit. Fluids 2021, 174, 105250. [Google Scholar] [CrossRef]
- Zizovic, I.; Tyrka, M.; Matya, K.; Moric, I.; Senerovic, L.; Trusek, A. Functional modification of cellulose acetate microfiltration membranes by supercritical solvent impregnation. Molecules 2021, 26, 411. [Google Scholar] [CrossRef]
- Nyman, J.; Svenarud, P.; van der Linden, J. Carbon dioxide de-airing in minimal invasive cardiac surgery, a new effective device. J. Cardiothorac. Surg. 2019, 14, 12. [Google Scholar] [CrossRef]
- Orihashi, K.; Ueda, T. “De-airing” in open heart surgery: Report from the CVSAP nation-wide survey and literature review. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 823–834. [Google Scholar] [CrossRef]
- Persson, M.; van der Linden, J. Wound ventilation with carbon dioxide: A simple method to prevent direct airborne contamination during cardiac surgery? J. Hosp. Infect. 2004, 56, 131–136. [Google Scholar] [CrossRef]
- Persson, M.; Flock, J.; van der Linden, J. Antiseptic wound ventilation with a gas diffuser: A new intraoperative method to prevent surgical wound infection? J. Hosp. Infect. 2003, 54, 294–299. [Google Scholar] [CrossRef]
- Baumann, M.; Cater, J.E. The effect of heated CO2 insufflation in minimising surgical wound contamination during open surgery. Ann. Biomed. Eng. 2018, 46, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- FDA, CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.515&SearchTerm=carvacrol (accessed on 20 May 2021).
- Nostro, A.; Blanco, A.R.M.; Cannatelli, A.; Enea, V.; Flamini, G.; Morelli, I.; Roccaro, A.S.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Leeke, G.A.; Santos, R.; King, M.B. Vapor-liquid equilibria for the carbon dioxide + carvacrol system at elevated pressures. J. Chem. Eng. Data 2001, 46, 541–545. [Google Scholar] [CrossRef]
- Milovanovic, S.; Radetic, M.; Misic, D.; Asanin, J.; Leontijevic, V.; Ivanovic, J.; Zizovic, I. High pressure modified cotton in wound dressing applications. In Cotton Fibers: Characteristics, Uses and Performance; Gordon, S., Abidi, N., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 117–205. [Google Scholar]
- Milovanovic, S.; Adamovic, T.; Aksentijevic, K.; Misic, D.; Ivanovic, J.; Zizovic, I. Cellulose acetate based material with antibacterial properties created by supercritical solvent impregnation. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Adamovic, T.; Milovanovic, S.; Markovic, D.; Zizovic, I. Impregnation of cellulose acetate films with carvacrol using supercritical carbon dioxide. Tehnika 2018, 73, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Dubois, J.; Grau, E.; Tassaing, T.; Dumon, M. On the CO2 sorption and swelling of elastomers by supercritical CO2 as studied by in situ high pressure FTIR microscopy. J. Supercrt. Fluids 2018, 131, 150–156. [Google Scholar] [CrossRef]
- Bonavoglia, B.; Storti, G.; Morbidelli, M.; Rajendran, A.; Mazzotti, M. Sorption and swelling of semicrystalline polymers.in supercritical CO2. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1531–1546. [Google Scholar] [CrossRef]
- Houben, M.; van Geijn, R.; Essen, M.; Borneman, Z.; Nijmeije, K. Supercritical CO2 permeation in glassy polyimide membranes. J. Memb. Sci. 2021, 620, 118922. [Google Scholar] [CrossRef]
- Marković, D.; Milovanović, S.; De Clerck, K.; Zizovic, I.; Stojanović, D.; Radetić, M. Development of material with strong antimicrobial activity by high pressure CO2 impregnation of polyamide nanofibers with thymol. J. CO2 Util. 2018, 26, 19–27. [Google Scholar] [CrossRef]
- Bertuola, M.; Fagali, N.; de Mele, F.L. Detection of carvacrol in essential oils by electrochemical polymerization. Heliyon 2020, 6, e03714. [Google Scholar] [CrossRef]
- Valderrama, A.S.S.; De Rojas, G.C. Traceability of active compounds of essential oils in antimicrobial food packaging using a chemometric method by ATR-FTIR. Am. J. Analyt. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef] [Green Version]
- Schulz, H.; Quilitzsch, R.; Krüger, H. Rapid evaluation and quantitative analysis of thyme, oregano and chamomile essential oils by ATR-IR and NIR spectroscopy. J. Mol. Struct. 2003, 661, 299–306. [Google Scholar] [CrossRef]
- Lopes, E.S.; Domingos, E.; Neves, R.S.; Romão, W.; Sousa, K.R.; Valaski, R.; Archanjo, B.S.; Souza, F.G., Jr.; Silva, A.M.; Kuznetsov, A.; et al. The role of intermolecular interactions in polyaniline/polyamide-6,6 pressure-sensitive blends studied by DFT and 1H NMR. Eur. Polym. J. 2016, 85, 588–604. [Google Scholar] [CrossRef]
- Pavliňák, D.; Hnilica, J.; Quade, A.; Schäfer, J.; Alberti, M.; Kudrle, V. Functionalisation and pore size control of electrospun PA6 nanofibres using a microwave jet plasma. Polym. Degrad. Stabil. 2014, 108, 48–55. [Google Scholar] [CrossRef]
- Kiakhani, M.S.; Safapour, S. Improvement of dyeing and antimicrobial properties of nylon fabrics modified using chitosan-poly(propylene imine) dendrimer hybrid. J. Ind. Eng. Chem. 2016, 32, 170–177. [Google Scholar] [CrossRef]
- Acero, E.H.; Ribitsch, D.; Rodriguez, R.D.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Greimel, K.J.; Schroeder, M.; Kandelbauer, A.; Heumann, S.; et al. Two-step enzymatic function of polyamide with phenolics. J. Mol. Catal. 2012, 79, 54–60. [Google Scholar] [CrossRef]
- Kaziran, S.G.; Martirosyan, G.G. Spectroscopy of polymer/drug formulations processed with supercritical fluids: In situ ATR–IR and Raman study of impregnation of ibuprofen into PVP. Int. J. Pharm. 2002, 232, 81–90. [Google Scholar] [CrossRef]
- Arnaudov, M.G.; Ivanova, B.B.; Dinkov, S.G. A reducing-difference IR-spectral study of 4-aminopyridine. Cent. Eur. J. Chem. 2004, 2, 589–597. [Google Scholar] [CrossRef]
- Good Vibrations with IR Spectroscopy. Available online: http://www1.udel.edu/chem/fox/IR_lectureNotes.pdf (accessed on 11 July 2021).
- Espinosa-Acosta, G.; Ramos-Jacques, A.L.; Molina, G.A.; Maya-Cornejo, J.; Esparza, R.; Hernandez-Martinez, A.R.; Sánchez-González, I.; Estevez, M. Stability analysis of anthocyanins using alcoholic extracts from black carrot (Daucus carota ssp. Sativus Var. Atrorubens Alef.). Molecules 2018, 23, 2744. [Google Scholar] [CrossRef] [Green Version]
- Milovanovic, S.; Markovic, D.; Aksentijevic, K.; Stojanovic, D.B.; Ivanovic, J.; Zizovic, I. Application of cellulose acetate for controlled release of thymol. Carbohyd. Polym. 2016, 147, 344–353. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical impregnation of cellulose acetate with thymol. J. Supercrit. Fluids 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Gottrup, F. Prevention of surgical-wound infections. N. Engl. J. Med. 2000, 342, 202–204. [Google Scholar] [CrossRef]













| Assignment | C | PA | PA + C | Ref. |
|---|---|---|---|---|
| C–H out-of-plane wagging | 811 cm−1 | - | 810 cm−1 | [29,30,31] |
| Carvacrol key characteristic peak | 864 cm−1 | - | 867 cm−1 | [29] |
| Carvacrol 1:2:4 substitution | 994 cm−1 | - | 995 cm−1 | [29,30,31] |
| Carvacrol ortho substitution | 1115 cm−1 | - | 1118 cm−1 | [29,30,31] |
| Carvacrol key characteristic peak | 1173 cm−1 | - | 1178 cm−1 | [29] |
| Carvacrol key characteristic peak | 1249 cm−1 | 1257 cm−1 | [29] | |
| Aliphatic –CH2/–CH3 stretching | 2958, 2926, and 2868 cm−1 | - | Overtone with CH2 stretching vibration in PA | [29,30,31] |
| –OH stretching vibration | 3378 cm−1 | - | Overtone with strong N–H stretching vibration in PA | [29,30,31] |
| N–H stretching vibration | - | 3299 and 3076 cm−1 | 3298 and 3065 cm−1 | [32,33] |
| CH2 asymmetric stretching vibrations | - | 2933 cm−1 | 2933 cm−1 | [32,33,34] |
| CH2 symmetric stretching vibrations | - | 2859 cm−1 | 2859 cm−1 | [32,33,34] |
| C=O stretching of amide | - | 1631 cm−1 | 1630 cm−1 | [32,33,34] |
| N–H bending of amide | - | 1536 cm−1 | 1534 cm−1 | [32,33,34] |
| O=C–N group bending | - | 689 cm−1 | 688 cm−1 | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, M.; Misic, D.; Trusek, A.; Zizovic, I. Polymeric Microfiltration Membranes Modification by Supercritical Solvent Impregnation—Potential Application in Open Surgical Wound Ventilation. Molecules 2021, 26, 4572. https://doi.org/10.3390/molecules26154572
Nowak M, Misic D, Trusek A, Zizovic I. Polymeric Microfiltration Membranes Modification by Supercritical Solvent Impregnation—Potential Application in Open Surgical Wound Ventilation. Molecules. 2021; 26(15):4572. https://doi.org/10.3390/molecules26154572
Chicago/Turabian StyleNowak, Mariusz, Dusan Misic, Anna Trusek, and Irena Zizovic. 2021. "Polymeric Microfiltration Membranes Modification by Supercritical Solvent Impregnation—Potential Application in Open Surgical Wound Ventilation" Molecules 26, no. 15: 4572. https://doi.org/10.3390/molecules26154572
APA StyleNowak, M., Misic, D., Trusek, A., & Zizovic, I. (2021). Polymeric Microfiltration Membranes Modification by Supercritical Solvent Impregnation—Potential Application in Open Surgical Wound Ventilation. Molecules, 26(15), 4572. https://doi.org/10.3390/molecules26154572