Added-Value Chemicals from Lignin Oxidation
Abstract
:1. Introduction
2. Lignin: Valorization, Structure, and Classification
3. Oxidative Depolymerization of Lignin
3.1. Phenolic Compounds from Lignin Oxidation
3.2. Dicarboxylic Acids from Lignin Oxidation
3.2.1. Non-Catalytic Harsh Oxidation
3.2.2. Catalytic Lignin Harsh Oxidation
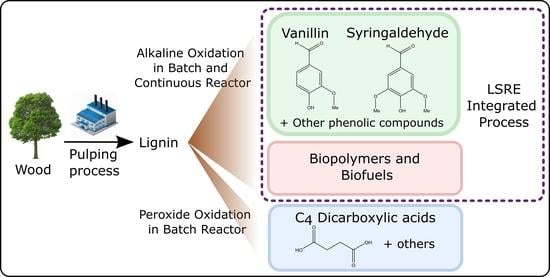
4. Integrated Process
Author Contributions
Funding
Conflicts of Interest
References
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Pinto, P.C.d.R.; Barreiro, M.F.; da Costa, C.A.E.; da Mota, M.I.F.; Fernandes, I. An Integrated Approach for Added-Value Products from Lignocellulosic Biorefineries; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-99312-6. [Google Scholar]
- Strassberger, Z.; Tanase, S.; Rothenberg, G. The pros and cons of lignin valorisation in an integrated biorefinery. RSC Adv. 2014, 4, 25310–25318. [Google Scholar] [CrossRef] [Green Version]
- Vishtal, A.G.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Lin, S.; Dence, C.; Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Bomgardner, M.M. Following many routes to naturally derived vanillin. Chem. Eng. News 2014, 92, 14. [Google Scholar]
- Huang, W.-B.; Du, C.-Y.; Jiang, J.-A.; Ji, Y.-F. Concurrent synthesis of vanillin and isovanillin. Res. Chem. Intermed. 2013, 39, 2849–2856. [Google Scholar] [CrossRef]
- Pinto, P.C.R.; Borges da Silva, E.A.; Rodrigues, A.E. Lignin as source of fine chemicals: Vanillin and syringaldehyde. In Biomass Conversion: The Interface of Biotechnology, Chemistry and Materials Science; Baskar, C., Baskar, S., Dhillon, R.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 381–420. ISBN 978-3-642-28418-2. [Google Scholar]
- Erofeev, Y.V.; Afanas’eva, V.L.; Glushkov, R.G. Synthetic routes to 3,4,5-trimethoxybenzaldehyde (review). Pharm. Chem. J. 2004, 24, 501–510. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Sriprasanthi, R.B.; Shamsudeen, S.; Adam, F.; Bhawani, S.A. A Concise Review of the Natural Existance, Synthesis, Properties, and Applications of Syringaldehyde. BioResources 2012, 7, 4377–4399. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chemie-Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Guo, M.; Zhang, X. Selective Conversion of Biorefinery Lignin into Dicarboxylic Acids. ChemSusChem 2014, 7, 412–415. [Google Scholar] [CrossRef]
- Gérardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.-C.M. Continuous Flow Upgrading of Selected C 2 –C 6 Platform Chemicals Derived from Biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef]
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries-Industrial Processes and Products: Status Quo and Future Directions. CHEMISTRY Int. 2008, 1–2. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I--Results of Screening for Potential Candidates from Sugars and Synthesis Gas.; National Renewable Energy Lab.: Golden, CO, USA, 2004; Volume 1. [Google Scholar]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Asgari, F.; Argyropoulos, D.S. Fundamentals of oxygen delignification. Part II. Functional group formation/elimination in residual kraft lignin. Can. J. Chem. 1998, 76, 1606–1615. [Google Scholar] [CrossRef]
- Korányi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass. Molecules 2020, 25, 2815. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Cheng, J.; Wang, D.; Yuan, T.-Q.; Song, G.; Barta, K. Downstream Processing Strategies for Lignin-First Biorefinery. ChemSusChem 2020, 13, 5199–5212. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; van Muyden, A.P.; Cui, X.; Fei, Z.; Yan, N.; Laurenczy, G.; Dyson, P.J. Lignin First: Confirming the Role of the Metal Catalyst in Reductive Fractionation. JACS Au 2021, 1, 729–733. [Google Scholar] [CrossRef]
- Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: The advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Henriksson, G. Chapter 9-Lignins: Major sources, structure and properties. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 201–224. ISBN 978-0-08-045316-3. [Google Scholar]
- Chan, J.C.; Paice, M.; Zhang, X. Enzymatic oxidation of lignin: Challenges and barriers toward practical applications. ChemCatChem 2020, 12, 401–425. [Google Scholar] [CrossRef]
- Constant, S.; Wienk, H.L.J.J.; Frissen, A.E.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.H.; Weckhuysen, B.M.; Huijgen, W.J.J.J.; Gosselink, R.J.A.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.A.E.E.; Pinto, P.C.R.; Rodrigues, A.E. Radar Tool for Lignin Classification on the Perspective of Its Valorization. Ind. Eng. Chem. Res. 2015, 54, 7580–7590. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Balakshin, M. Industrial Lignins. In Bioenergy Research: Advances and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. ISBN 9780444595614. [Google Scholar]
- Costa, C.A.E.; Coleman, W.; Dube, M.; Rodrigues, A.E.; Pinto, P.C.R. Assessment of key features of lignin from lignocellulosic crops: Stalks and roots of corn, cotton, sugarcane, and tobacco. Ind. Crops Prod. 2016, 92, 136–148. [Google Scholar] [CrossRef]
- Costa, C.A.E.E.; Pinto, P.C.R.R.; Rodrigues, A.E. Lignin fractionation from E. Globulus kraft liquor by ultrafiltration in a three stage membrane sequence. Sep. Purif. Technol. 2018, 192, 140–151. [Google Scholar] [CrossRef]
- Costa, C.A.E.E.; Pinto, P.C.R.; Rodrigues, A.E. Evaluation of chemical processing impact on E. globulus wood lignin and comparison with bark lignin. Ind. Crops Prod. 2014, 61, 479–491. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Evstigneyev, E.I. Selective depolymerization of lignin: Assessment of yields of monomeric products. J. Wood Chem. Technol. 2018, 38, 409–415. [Google Scholar] [CrossRef]
- Lange, H.; Decina, S.; Crestini, C. Oxidative upgrade of lignin-Recent routes reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Liu, X.; Bouxin, F.P.; Fan, J.; Budarin, V.L.; Hu, C.; Clark, J.H. Recent advances in the catalytic depolymerization of lignin towards phenolic chemicals: A review. ChemSusChem 2020, 13, 4296–4317. [Google Scholar] [CrossRef]
- Patil, V.; Adhikari, S.; Cross, P.; Jahromi, H. Progress in the solvent depolymerization of lignin. Renew. Sustain. Energy Rev. 2020, 133, 110359. [Google Scholar] [CrossRef]
- Rodrigues Pinto, P.C.; Borges da Silva, E.A.; Rodrigues, A.E. Insights into Oxidative Conversion of Lignin to High-Added-Value Phenolic Aldehydes. Ind. Eng. Chem. Res. 2011, 50, 741–748. [Google Scholar] [CrossRef]
- Vangeel, T.; Schutyser, W.; Renders, T.; Sels, B.F. Perspective on Lignin Oxidation: Advances, Challenges, and Future Directions. Top. Curr. Chem. 2018, 376, 30. [Google Scholar] [CrossRef]
- Araújo, J.D.P.; Grande, C.A.; Rodrigues, A.E. Vanillin production from lignin oxidation in a batch reactor. Chem. Eng. Res. Des. 2010, 88, 1024–1032. [Google Scholar] [CrossRef]
- Ma, R.; Xu, Y.; Zhang, X. Catalytic Oxidation of Biorefinery Lignin to Value-added Chemicals to Support Sustainable Biofuel Production. ChemSusChem 2015, 8, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Mathias, A.L.; Rodrigues, A.E. Production of vanillin by oxidation of pine kraft lignins with oxygen. Holzforschung 1995, 49, 273–278. [Google Scholar] [CrossRef]
- Pinto, P.C.R.; Costa, C.E.; Rodrigues, A.E. Oxidation of Lignin from Eucalyptus globulus Pulping Liquors to Produce Syringaldehyde and Vanillin. Ind. Eng. Chem. Res. 2013, 52, 4421–4428. [Google Scholar] [CrossRef]
- Villar, J.C.C.; Caperos, A.; García-Ochoa, F. Oxidation of hardwood kraft-lignin to phenolic derivatives with oxygen as oxidant. Wood Sci. Technol. 2001, 35, 245–255. [Google Scholar] [CrossRef]
- Schutyser, W.; Kruger, J.S.; Robinson, A.M.; Katahira, R.; Brandner, D.G.; Cleveland, N.S.; Mittal, A.; Peterson, D.J.; Meilan, R.; Román-Leshkov, Y.; et al. Revisiting alkaline aerobic lignin oxidation. Green Chem. 2018, 20, 3828–3844. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Fargues, C.; Mathias, Á.; Rodrigues, A. Kinetics of Vanillin Production from Kraft Lignin Oxidation. Ind. Eng. Chem. Res. 1996, 35, 28–36. [Google Scholar] [CrossRef]
- Tarabanko, V.; Petukhov, D. Study of mechanism and improvement of the process of oxidative cleavage of ligins into the aromatic aldehydes. Chem. Sustain. Dev. 2003, 11, 655–667. [Google Scholar]
- Tarabanko, V.E.; Fomova, N.A.; Kuznetsov, B.N.; Ivanchenko, N.M.; Kudryashev, A. V On the mechanism of vanillin formation in the catalytic oxidation of lignin with oxygen. React. Kinet. Catal. Lett. 1995, 55, 161–170. [Google Scholar] [CrossRef]
- Costa, C.A.E. Vanillin and Syringaldehyde from Side Streams of Pulp and Paper Industries and Biorefineries. Ph.D. Thesis, University of Porto, Porto, Portugal, 2017. [Google Scholar]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, M.; Paice, M.G. Routes to potential bioproducts from lignocellulosic biomass lignin and hemicelluloses. BioEnergy Res. 2011, 4, 246–257. [Google Scholar] [CrossRef]
- Santos, S.G.; Marques, A.P.; Lima, D.L.D.; Evtuguin, D.V.; Esteves, V.I. Kinetics of Eucalypt lignosulfonate oxidation to aromatic aldehydes by oxygen in alkaline medium. Ind. Eng. Chem. Res. 2011, 50, 291–298. [Google Scholar] [CrossRef] [Green Version]
- da Silva, E.A.B.; Zabkova, M.; Araújo, J.D.; Cateto, C.A.; Barreiro, M.F.; Belgacem, M.N.; Rodrigues, A.E. An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Araújo, J.D.P. Production of Vanillin from Lignin Present in the Kraft Black Liquor of the Pulp and Paper Industry. Ph.D. Thesis, University of Porto, Porto, Portugal, 2008. [Google Scholar]
- Araújo, J.D.P.; Grande, C.A.; Rodrigues, A.E. Structured packed bubble column reactor for continuous production of vanillin from Kraft lignin oxidation. Catal. Today 2009, 147, S330–S335. [Google Scholar] [CrossRef]
- Gomes, E.D.; Rodrigues, A.E. Lignin biorefinery: Separation of vanillin, vanillic acid and acetovanillone by adsorption. Sep. Purif. Technol. 2019, 216, 92–101. [Google Scholar] [CrossRef]
- Casimiro, F.M.; Costa, C.A.E.E.; Botelho, C.M.; Barreiro, M.F.; Rodrigues, A.E. Kinetics of oxidative degradation of lignin-based phenolic compounds in batch reactor. Ind. Eng. Chem. Res. 2019, 58, 16442–16449. [Google Scholar] [CrossRef] [Green Version]
- Gomes, E.D. Development of a Continuous Process for the Production of Vanillin and Syringaldehyde from Kraft Black Liquor. Ph.D. Thesis, University of Porto, Porto, Portugal, 2019. [Google Scholar]
- Fargues, C.; Mathias, Á.; Silva, J.; Rodrigues, A. Kinetics of vanillin oxidation. Chem. Eng. Technol. 1996, 19, 127–136. [Google Scholar] [CrossRef]
- Pacek, A.W.; Ding, P.; Garrett, M.; Sheldrake, G.; Nienow, A.W. Catalytic conversion of sodium lignosulfonate to vanillin: Engineering aspects. Part 1. Effects of processing conditions on vanillin yield and selectivity. Ind. Eng. Chem. Res. 2013, 52, 8361–8372. [Google Scholar] [CrossRef]
- Pinto, P.C.R.R.; da Silva, E.A.B.B.; Rodrigues, A.E. Comparative Study of Solid-Phase Extraction and Liquid−Liquid Extraction for the Reliable Quantification of High Value Added Compounds from Oxidation Processes of Wood-Derived Lignin. Ind. Eng. Chem. Res. 2010, 49, 12311–12318. [Google Scholar] [CrossRef]
- Ma, R.; Guo, M.; Zhang, X. Recent advances in oxidative valorization of lignin. Catal. Today 2018, 302, 50–60. [Google Scholar] [CrossRef]
- Litsanov, B.; Brocker, M.; Oldiges, M.; Bott, M. Succinic Acid. In Bioprocessing of Renewable Resources to Commodity Bioproducts; Bisaria, V.S., Kondo, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 435–472. [Google Scholar]
- de Jong, E.; Jungmeier, G. Biorefinery Concepts in Comparison to Petrochemical Refineries. In Industrial Biorefineries and White Biotechnology; Elsevier, B.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–33. ISBN 9780444634535. [Google Scholar]
- Cok, B.; Tsiropoulos, I.; Roes, A.L.; Patel, M.K. Succinic acid production derived from carbohydrates: An energy and greenhouse gas assessment of a platform chemical toward a bio-based economy. Biofuels Bioprod. Biorefining 2014, 8, 16–29. [Google Scholar] [CrossRef]
- West, T.P. Microbial Production of Malic Acid from Biofuel-Related Coproducts and Biomass. Fermentation 2017, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Martin-Dominguez, V.; Estevez, J.; Ojembarrena, F.D.B.; Santos, V.E.; Ladero, M. Fumaric Acid Production: A Biorefinery Perspective. Fermentation 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, I.; Rokem, J.S. Fumaric Acid Biosynthesis and Accumulation. Bioprocess. Renew. Resour. Commod. Bioprod. 2014, 409–434. [Google Scholar] [CrossRef]
- Demesa, A.G.; Laari, A.; Turunen, I.; Sillanpää, M. Alkaline Partial Wet Oxidation of Lignin for the Production of Carboxylic Acids. Chem. Eng. Technol. 2015, 38, 2270–2278. [Google Scholar] [CrossRef]
- Cabral Almada, C.; Kazachenko, A.; Fongarland, P.; Da Silva Perez, D.; Kuznetsov, B.N.; Djakovitch, L. Oxidative depolymerization of lignins for producing aromatics: Variation of botanical origin and extraction methods. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Figueirêdo, M.B.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J. Valorization of Pyrolysis Liquids: Ozonation of the Pyrolytic Lignin Fraction and Model Components. ACS Sustain. Chem. Eng. 2019, 7, 4755–4765. [Google Scholar] [CrossRef]
- Evtuguin, D.; Robert, D. The detection of muconic acid type structures in oxidized lignins by13C NMR spectroscopy. Wood Sci. Technol. 1997, 31, 423–431. [Google Scholar] [CrossRef]
- Quesada, J.; Rubio, M.; Gómez, D. Ozonation of Lignin Rich Solid Fractions from Corn Stalks. J. Wood Chem. Technol. 1999, 19, 115–137. [Google Scholar] [CrossRef]
- Guélou, E.; Barrault, J.; Fournier, J.; Tatibouët, J.M. Active iron species in the catalytic wet peroxide oxidation of phenol over pillared clays containing iron. Appl. Catal. B Environ. 2003, 44, 1–8. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K.H. Catalytic oxidation of lignin in solvent systems for production of renewable chemicals: A review. Polymers 2017, 9, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, S.K.; Tardio, J.; Prasad, J.; Föger, K.; Akolekar, D.B.; Grocott, S.C. Wet oxidation and catalytic wet oxidation. Ind. Eng. Chem. Res. 2006, 45, 1221–1258. [Google Scholar] [CrossRef]
- Yin, G.; Jin, F.; Yao, G.; Jing, Z. Hydrothermal Conversion of Catechol into Four-Carbon Dicarboxylic Acids. Ind. Eng. Chem. Res. 2015, 54, 68–75. [Google Scholar] [CrossRef]
- Xiang, Q.; Lee, Y.Y. Oxidative cracking of precipitated hardwood lignin by hydrogen peroxide. Appl. Biochem. Biotechnol. 2000, 84–86, 153–162. [Google Scholar] [CrossRef]
- Suzuki, H.; Cao, J.; Jin, F.; Kishita, A.; Enomoto, H.; Moriya, T. Wet oxidation of lignin model compounds and acetic acid production. J. Mater. Sci. 2006, 41, 1591–1597. [Google Scholar] [CrossRef]
- Ma, R.; Guo, M.; Lin, K.T.; Hebert, V.R.; Zhang, J.; Wolcott, M.P.; Quintero, M.; Ramasamy, K.K.; Chen, X.; Zhang, X. Peracetic Acid Depolymerization of Biorefinery Lignin for Production of Selective Monomeric Phenolic Compounds. Chem.-A Eur. J. 2016, 22, 10884–10891. [Google Scholar] [CrossRef]
- Hasegawa, I.; Inoue, Y.; Muranaka, Y.; Yasukawa, T.; Mae, K. Selective Production of Organic Acids and Depolymerization of Lignin by Hydrothermal Oxidation with Diluted Hydrogen Peroxide. Energy Fuels 2011, 25, 791–796. [Google Scholar] [CrossRef]
- Vega-Aguilar, C.A.; Barreiro, M.F.; Rodrigues, A.E. Effect of Methoxy Substituents on Wet Peroxide Oxidation of Lignin and Lignin Model Compounds: Understanding the Pathway to C 4 Dicarboxylic Acids. Ind. Eng. Chem. Res. 2021, 60, 3543–3553. [Google Scholar] [CrossRef]
- Rovio, S.; Kallioinen, A.; Tamminen, T.; Hakola, M.; Leskelä, M.; Siika-ahoa, M. Catalysed alkaline oxidation as a wood fractionation technique. BioResources 2012, 7, 756–776. [Google Scholar]
- Wu, G.; Heitz, M. Catalytic mechanism of Cu2+ and Fe3+ in alkaline O2 oxidation of lignin. J. Wood Chem. Technol. 1995, 15, 189–202. [Google Scholar] [CrossRef]
- Faisal, I. Oxidation of Phenolic Wastewater by Fenton’s Reagent. Iraqi J. Chem. Pet. Eng. 2009, 10, 1–5. [Google Scholar]
- Kang, J.; Irmak, S.; Wilkins, M. Conversion of lignin into renewable carboxylic acid compounds by advanced oxidation processes. Renew. Energy 2019, 135, 951–962. [Google Scholar] [CrossRef]
- Zeng, J.; Yoo, C.G.; Wang, F.; Pan, X.; Vermerris, W.; Tong, Z. Biomimetic fenton-catalyzed lignin depolymerization to high-value aromatics and dicarboxylic acids. ChemSusChem 2015, 8, 861–871. [Google Scholar] [CrossRef]
- Ansaloni, S.; Russo, N.; Pirone, R. Wet Air Oxidation of Industrial Lignin Case Study: Influence of the Dissolution Pretreatment and Perovskite-type Oxides. Waste Biomass Valorization 2018, 9, 2165–2179. [Google Scholar] [CrossRef]
- Cronin, D.J.; Zhang, X.; Bartley, J.; Doherty, W.O.S.S. Lignin Depolymerization to Dicarboxylic Acids with Sodium Percarbonate. ACS Sustain. Chem. Eng. 2017, 5, 6253–6260. [Google Scholar] [CrossRef] [Green Version]
- Bi, Z.; Li, Z.; Yan, L. Catalytic oxidation of lignin to dicarboxylic acid over the CuFeS2 nanoparticle catalyst. Green Process. Synth. 2018, 7, 306–315. [Google Scholar] [CrossRef]
- Lotfi, S.; Boffito, D.C.; Patience, G.S. Gas-Phase Partial Oxidation of Lignin to Carboxylic Acids over Vanadium Pyrophosphate and Aluminum-Vanadium-Molybdenum. ChemSusChem 2015, 8, 3424–3432. [Google Scholar] [CrossRef]
- Lotfi, S.; Boffito, D.C.; Patience, G.S. Gas–solid conversion of lignin to carboxylic acids. React. Chem. Eng. 2016, 1, 397–408. [Google Scholar] [CrossRef]
- Clerici, M.G. The Activity of Titanium Silicalite-1 (TS-1): Some Considerations on Its Origin. Kinet. Catal. 2015, 56, 453–458. [Google Scholar] [CrossRef]
- Gamba, A.; Tabacchi, G.; Fois, E. TS-1 from First Principles. J. Phys. Chem. A 2009, 113, 15006–15015. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Peng, X.; Zhang, Y.; Wang, B.; Lin, M.; Zhu, B.; Luo, Y.; Shu, X. Environmental-Friendly Catalytic Oxidation Processes Based on Hierarchical Titanium Silicate Zeolites at SINOPEC. In Green Chemical Processing and Synthesis; Karamé, I., Ed.; InTech: London, UK, 2017; pp. 119–150. [Google Scholar]
- Su, J.; Yang, L.; Liu, R.N.; Lin, H. Low-temperature oxidation of guaiacol to maleic acid over TS-1 catalyst in alkaline aqueous H2O2 solutions. Chinese J. Catal. 2014, 35, 622–630. [Google Scholar] [CrossRef]
- Rodenas, Y.; Mariscal, R.; Fierro, J.L.G.G.; Martín Alonso, D.; Dumesic, J.A.; López Granados, M. Improving the production of maleic acid from biomass: TS-1 catalysed aqueous phase oxidation of furfural in the presence of γ-valerolactone. Green Chem. 2018, 20, 2845–2856. [Google Scholar] [CrossRef]
- Alba-Rubio, A.C.; Fierro, J.L.G.; León-Reina, L.; Mariscal, R.; Dumesic, J.A.; López Granados, M. Oxidation of furfural in aqueous H2O2 catalysed by titanium silicalite: Deactivation processes and role of extraframework Ti oxides. Appl. Catal. B Environ. 2017, 202, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Vega-Aguilar, C.A.; Barreiro, M.F.; Rodrigues, A.E. Catalytic wet peroxide oxidation of vanillic acid as a lignin model compound towards the renewable production of dicarboxylic acids. Chem. Eng. Res. Des. 2020, 159, 115–124. [Google Scholar] [CrossRef]
- Vega-Aguilar, C.A.; Barreiro, M.F.; Rodrigues, A.E. Valorisation of lignin into C4 dicarboxylic acids by catalytic wet peroxide oxidation using TS-1 catalyst. Ind. Crops Prod. 2021. Unpublished work. [Google Scholar]
- Mota, I.F.; Pinto, P.R.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Downstream processing of an oxidized industrial kraft liquor by membrane fractionation for vanillin and syringaldehyde recovery. Sep. Purif. Technol. 2018, 197, 360–371. [Google Scholar] [CrossRef]
- Zabkova, M.; da Silva, E.A.B.; Rodrigues, A.E. Recovery of vanillin from lignin/vanillin mixture by using tubular ceramic ultrafiltration membranes. J. Memb. Sci. 2007, 301, 221–237. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Brochier-Salon, M.C.; Thielemans, W.; Belgacem, M.N. Lignins as macromonomers for polyurethane synthesis: A comparative study on hydroxyl group determination. J. Appl. Polym. Sci. 2008, 109, 3008–3017. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E. Monitoring of lignin-based polyurethane synthesis by FTIR-ATR. Ind. Crops Prod. 2008, 27, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization Study of Lignin Oxypropylation in View of the Preparation of Polyurethane Rigid Foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589. [Google Scholar] [CrossRef]
- Zabkova, M.; Otero, M.; Minceva, M.; Zabka, M.; Rodrigues, A.E. Separation of synthetic vanillin at different pH onto polymeric adsorbent Sephabeads SP206. Chem. Eng. Process. Process. Intensif. 2006, 45, 598–607. [Google Scholar] [CrossRef]
- Gomes, E.D.D.; Mota, M.I.I.; Rodrigues, A.E.E. Fractionation of acids, ketones and aldehydes from alkaline lignin oxidation solution with SP700 resin. Sep. Purif. Technol. 2018, 194, 256–264. [Google Scholar] [CrossRef]
- Mota, M.I.F.; Pinto, P.C.R.; Loureiro, J.M.; Rodrigues, A.E. Adsorption of vanillin and syringaldehyde onto a macroporous polymeric resin. Chem. Eng. J. 2016, 288, 869–879. [Google Scholar] [CrossRef]












| Vanillin, % w/w lignin * | Syringaldehyde, % w/w lignin * | |||
|---|---|---|---|---|
| NO ** | Alkaline Oxid. | NO ** | Alkaline Oxid. | |
| LInAT 1,2 | 9.3 | 3.4 | - | - |
| LWest 1 | 12.1 | 4.4 | - | - |
| LBoostS 1 | 11.1 | 3.1 | - | - |
| LOrgs 1 | 4.8 | 1.2 | 13.2 | 2.5 |
| KL 3 | 2.9 | 0.73 | 12.5 | 1.9 |
| KLlig 3 | 3.4 | 1.2 | 13.6 | 2.8 |
| EKL 3 | 2.2 | 0.71 | 9.2 | 1.4 |
| EKLlig 3 | 2.5 | 0.82 | 9.5 | 2.0 |
| HTEKL 3 | 3.4 | 0.54 | 9.8 | 1.5 |
| HTEKLlig 3 | 2.6 | 0.94 | 9.8 | 2.0 |
| SL 3 | 2.5 | 1.5 | 11.3 | 3.3 |
| LTobObut 4 | 2.8 | 0.74 | 2.5 | 0.34 |
| LTobOethan 4 | 7.2 | 1.2 | 4.8 | 0.94 |
| LCelbi | 1.7 | 0.81 | 9.5 | 2.1 |
| Lignin | Catalyst | Acid Yields (wt%) | ||||
|---|---|---|---|---|---|---|
| Succinic | Malic | Maleic | Fumaric | Tartaric | ||
| ALK | No | 1.6 | 1.0 | n.d. | n.d. | n.d. |
| TS-1 | 5.8 | 6.6 | 0.1 | 0.04 | 0.9 | |
| IAT | No | 2.4 | 4.8 | n.d. | n.d. | n.d. |
| TS-1 | 11.3 | 10.1 | 0.1 | 0.06 | n.d. | |
| EOL | No | 6.9 | 22.6 | n.d. | n.d. | n.d. |
| TS-1 | 9.7 | 19.5 | 0.1 | 0.04 | n.d. | |
| EKL | No | 0.6 | 3.6 | n.d. | n.d. | n.d. |
| TS-1 | 7.6 | 5.5 | n.d. | n.d. | n.d. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.A.E.; Vega-Aguilar, C.A.; Rodrigues, A.E. Added-Value Chemicals from Lignin Oxidation. Molecules 2021, 26, 4602. https://doi.org/10.3390/molecules26154602
Costa CAE, Vega-Aguilar CA, Rodrigues AE. Added-Value Chemicals from Lignin Oxidation. Molecules. 2021; 26(15):4602. https://doi.org/10.3390/molecules26154602
Chicago/Turabian StyleCosta, Carina A. Esteves, Carlos A. Vega-Aguilar, and Alírio E. Rodrigues. 2021. "Added-Value Chemicals from Lignin Oxidation" Molecules 26, no. 15: 4602. https://doi.org/10.3390/molecules26154602
APA StyleCosta, C. A. E., Vega-Aguilar, C. A., & Rodrigues, A. E. (2021). Added-Value Chemicals from Lignin Oxidation. Molecules, 26(15), 4602. https://doi.org/10.3390/molecules26154602