Supercritical CO2 Extraction as a Tool to Isolate Anti-Inflammatory Sesquiterpene Lactones from Cichorium intybus L. Roots
Abstract
:1. Introduction
2. Results and Discussion
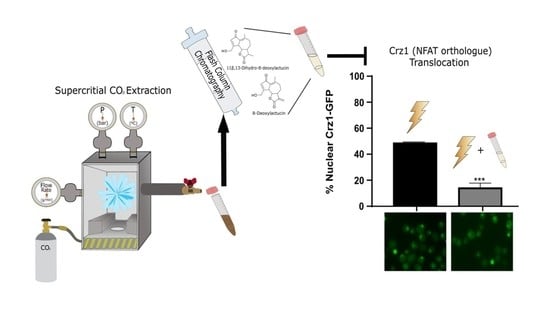
2.1. Supercritical CO2 Extraction of SLs
2.2. Conventional Solid–Liquid Extraction of SL
2.3. Fractionation of Cichorium intybus L. Extract
2.4. SFE Extract Potential Anti-Inflammatory Activity
2.5. Purified Fraction Anti-Inflammatory Potential
3. Materials and Methods
3.1. Chemicals
3.2. Raw Material
3.3. Extraction and Fractionation Procedures
3.3.1. Supercritical CO2 Extractions
3.3.2. Experimental Design Analysis/Statistical Analysis
3.3.3. Conventional Solid–Liquid Extraction
3.3.4. Flash Column Chromatography Purification
3.4. SL Analysis
3.4.1. Quantification of SL by HPLC with Diode-Array Detector (HPLC-DAD)
3.4.2. Identification of SL by Liquid Chromatography–Mass Spectrometry (LC-MS)
3.5. Anti-Inflammatory Evaluation
3.5.1. Saccharomyces Cerevisiae Strains and Growth Conditions
3.5.2. Cell Viability Assays
3.5.3. Measurement of Reporter Gene Activity
3.5.4. Fluorescence Microscopy
3.6. Statistical Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Bais, H.P.; Ravishankar, G.A. Review Cichorium intybus L–cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J. Sci. Food Agric. 2001, 484, 467–484. [Google Scholar] [CrossRef]
- Al-snafi, P.A.E.; Medicine, C. Medical importance of Cichorium intybus–A review. Iosr J. Pharm. 2016, 6, 41–56. [Google Scholar]
- Siddhan, N.; Kumari, B.D.R. Phytochemical and Antibacterial Studies of Chicory (Cichorium intybus L.)-A Multipurpose Medicinal Plant Phytochemical and Antibacterial Studies of Chicory. Adv. Biol. Res. 2014, 1, 17–21. [Google Scholar]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evidence-based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Mares, D.; Romagnoli, C.; Tosi, B.; Andreotti, E.; Chillemi, G.; Poli, F. Chicory extracts from Cichorium intybus L. as potential antifungals. Mycopathologia 2005, 160, 85–92. [Google Scholar] [CrossRef]
- Mai, F.; Glomb, M.A. Structural and Sensory Characterization of Novel Sesquiterpene Lactones from Iceberg Lettuce. J. Agric. Food Chem. 2016, 64, 295–301. [Google Scholar] [CrossRef]
- Bischoff, T.A.; Kelley, C.J.; Karchesy, Y.; Laurantos, M.; Nguyen-Dinh, P.; Arefi, A.G. Antimalarial activity of Lactucin and Lactucopicrin: Sesquiterpene lactones isolated from Cichorium intybus L. J. Ethnopharmacol. 2004, 95, 455–457. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Peña-Espinoza, M.; Valente, A.H.; Thamsborg, S.M.; Simonsen, H.T.; Boas, U.; Enemark, H.L.; López-Muñoz, R.; Williams, A.R. Antiparasitic activity of chicory (Cichorium intybus) and its natural bioactive compounds in livestock: A review. Parasites Vectors 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Y.S.; Lebda, M.A.; Hassinin, M.; Neoman, S.A. Chicory (Cichorium intybus L.) root extract regulates the oxidative status and antioxidant gene transcripts in CCl4-induced hepatotoxicity. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Ripoll, C.; Schmidt, B.M.; Ilic, N.; Poulev, A.; Dey, M.; Kurmukov, A.; Raskin, I. Anti-inflammatory Effects of a Sesquiterpene Lactone Extract from Chicory (Cichorium intybus L.) Roots. Nat. Prod. Commun. 2007, 2, 717–722. [Google Scholar] [CrossRef]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient–Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef] [PubMed]
- van Beek, T.A.; Maas, P.; de Groot, A.; King, B.M.; Leclercq, E.; Voragen, A.G.L. Bitter Sesquiterpene Lactones from Chicory Roots. J. Agric. Food Chem. 1990, 38, 1035–1038. [Google Scholar] [CrossRef]
- Malarz, J.; Stojakowska, A.; Kisiel, W. Sesquiterpene lactones in a hairy root culture of Cichorium intybus. Z. Für Nat. C 2014, 57, 11–12. [Google Scholar] [CrossRef] [Green Version]
- Cavin, C.; Delannoy, M.; Malnoe, A.; Debefve, E.; Touché, A.; Courtois, D.; Schilter, B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Commun. 2005, 327, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, F.; D’Antuono, L.F. An update procedure for an effective and simultaneous extraction of sesquiterpene lactones and phenolics from chicory. Food Chem. 2012, 135, 243–250. [Google Scholar] [CrossRef]
- Foster, J.G.; Clapham, W.M.; Belesky, D.P.; Labreveux, M.; Hall, M.H.; Sanderson, M.A. Influence of cultivation site on sesquiterpene lactone composition of forage chicory (Cichorium intybus L.). J. Agric. Food Chem. 2006, 54, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Stojakowska, A.; Malarz, J.; Dolezalova, I.; Ales Lebeda, W.K. Systematic implications of sesquiterpene lactones in Lactuca species. Biochem. Syst. Ecol. 2009, 37, 174–179. [Google Scholar] [CrossRef]
- Delgado, G.; Garcia, P.E.; Roldan, R.I.; Bye, R.; Linares, E. New Eremophilane Sesquiterpene Lactones from the Roots of the Medicinal Plant Roldana sessilifolia (Asteracea). Nat. Prod. Lett. 2006, 37–41. [Google Scholar] [CrossRef]
- David, J.P.; Santos, A.J.D.O.; Guedes, M.L.S.; David, J.M.; Chai, H.; Pezzuto, J.M.; Angerhofer, C.K.; Cordell, G.A. Sesquiterpene Lactones from ambrosia artemisiaefolia (Asteraceae). Pharm. Biol. 1999, 37, 165–168. [Google Scholar] [CrossRef]
- Sakamoto, H.T.; Laudares, E.P.; Crotti, A.E.; Lopes, N.P.; Vichnewski, W.; Lopes, J.L.; Heleno, V.C. Sesquiterpenes lactones and flavonoids from Eremanthus argenteus (Asteraceae). Nat. Prod. Commun. 2010, 5, 681–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A. 2007, 1163, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trac-Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.G.; Meireles, M.A.A. Supercritical Fluid Extraction of Bioactive Compounds: Fundamentals, Applications and Economic Perspectives. Food Bioprocess. Tech. 2010, 340–372. [Google Scholar] [CrossRef]
- Kisiel, W.; Zielin, K. Guaianolides from Cichorium intybus and structure revision of Cichorium sesquiterpene lactones. Phytochemistry 2001, 57, 523–527. [Google Scholar] [CrossRef]
- Hailes, H.C. Reaction Solvent Selection: The Potential of Water as a Solvent for Organic Transformations Abstract. Org. Process. Res. Dev. 2007, 11, 521–527. [Google Scholar] [CrossRef]
- Arbera, Ä.S.; Erreres, F.E.F. Lettuce and Chicory Byproducts as a Source of Antioxidant Phenolic Extracts. J. Agric. Food. Chem. 2004, 5109–5116. [Google Scholar] [CrossRef]
- Graziani, G.; Ferracane, R.; Sambo, P.; Santagata, S.; Nicoletto, C.; Fogliano, V. Profiling chicory sesquiterpene lactones by high resolution mass spectrometry. Food Res. Int. 2015, 67, 193–198. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Allwood, J.W.; Carregosa, D.; Marques, D.; Sungurtas, J.; McDougall, G.J.; Menezes, R.; Matias, A.A.; Stewart, D.; et al. Assessing the intestinal permeability and anti-inflammatory potential of sesquiterpene lactones from chicory. Nutrients 2020, 12, 3547. [Google Scholar] [CrossRef]
- Saadane, A.; Masters, S.; DiDonato, J.; Li, J.; Berger, M. Parthenolide inhibits IkappaB kinase, NF-kappaB activation, and inflammatory response in cystic fibrosis cells and mice. Am. J. Respir Cell Mol. Biol 2007, 36, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Li, H.; Rao, A.; Hogan, P.G. Inhibition of the calcineurin-NFAT interaction by small organic molecules reflects binding at an allosteric site. J. Biol. Chem. 2005, 280, 37698–37706. [Google Scholar] [CrossRef] [Green Version]
- King, M.M. Modification of the calmodulin-stimulated phosphatase, calcineurin, by sulfhydryl reagents. J. Biol. Chem. 1986, 261, 4081–4084. [Google Scholar] [CrossRef]
- Thormann, U.; Hänggi, R.; Kreuter, M.; Imanidis, G. Membrane transport of nobilin conjugation products and use of the extract of Chamomillae romanae flos influence absorption of nobilin in the Caco-2 model. Eur. J. Pharm. Sci. 2015, 70, 92–106. [Google Scholar] [CrossRef]
- Araki, Y.; Wu, H.; Kitagaki, H.; Akao, T.; Takagi, H.; Shimoi, H. Ethanol stress stimulates the Ca2+-mediated calcineurin/Crz1 pathway in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2009, 107, 1–6. [Google Scholar] [CrossRef]
- Rodrigues, L.; Silva, I.; Poejo, J.; Serra, A.T.; Matias, A.A.; Simplício, A.L.; Bronze, M.R.; Duarte, C.M.M. Recovery of antioxidant and antiproliferative compounds from watercress using pressurized fluid extraction. RSC Adv. 2016, 6, 30905–30918. [Google Scholar] [CrossRef]
- Khuri, I.; Mukhopadhyay, S. Response surface methodology. Wires 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Ba, D.; Boyaci, I.H. Modeling and optimization i: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Almeida, M.; Erthal, R.; Padua, E.; Silveira, L.; Am, L. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Miller, J. Experiments in molecular genetics. Astrophys. Space Sci. 1972. [Google Scholar]
- Menezes, R.; Foito, A.; Jardim, C.; Costa, I.; Garcia, G.; Rosado-Ramos, R.; Freitag, S.; Alexander, C.J.; Outeiro, T.F.; Stewart, D.; et al. Bioprospection of Natural Sources of Polyphenols with Therapeutic Potential for Redox-Related Diseases. Antioxid 2020, 9, 789. [Google Scholar] [CrossRef]










| Levels | ||||
|---|---|---|---|---|
| Variable, unit | −1 | 0 | 1 | |
| DOE 1 | Temperature, °C | 40 | 60 | 80 |
| Pressure, bar | 100 | 325 | 550 | |
| DOE 2 | Temperature, °C | 25 | 33 | 40 |
| Co-solvent, % EtOH | 10 | 25 | 40 | |
| Flow rate, g/min | 10 | 20 | 30 | |
| Polynomial Model Equations | R2 | Radj2 |
|---|---|---|
| MY = 1.232 + 0.936A + 0.403B − 0.124A2 + 0.285BC | 0.99 | 0.98 |
| 11β,13-dihydrolactucin = 4.2 + 0.748A − 6.096B − 1.519C + 4.01B2 | 0.98 | 0.97 |
| Lactucin = 6.262 + 1.046A − 9.261B − 2.155C + 6.088B2 | 0.98 | 0.98 |
| 11β,13-dihydrolactucopicrin = 1.688 − 0.876B | 0.90 | 0.87 |
| Lactucopicrin = 5.564 − 4.293B − 1.271C + 1.236B2 | 0.96 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baixinho, J.P.; Anastácio, J.D.; Ivasiv, V.; Cankar, K.; Bosch, D.; Menezes, R.; de Roode, M.; dos Santos, C.N.; Matias, A.A.; Fernández, N. Supercritical CO2 Extraction as a Tool to Isolate Anti-Inflammatory Sesquiterpene Lactones from Cichorium intybus L. Roots. Molecules 2021, 26, 2583. https://doi.org/10.3390/molecules26092583
Baixinho JP, Anastácio JD, Ivasiv V, Cankar K, Bosch D, Menezes R, de Roode M, dos Santos CN, Matias AA, Fernández N. Supercritical CO2 Extraction as a Tool to Isolate Anti-Inflammatory Sesquiterpene Lactones from Cichorium intybus L. Roots. Molecules. 2021; 26(9):2583. https://doi.org/10.3390/molecules26092583
Chicago/Turabian StyleBaixinho, João P., José D. Anastácio, Viktoriya Ivasiv, Katarina Cankar, Dirk Bosch, Regina Menezes, Matthew de Roode, Cláudia Nunes dos Santos, Ana A. Matias, and Naiara Fernández. 2021. "Supercritical CO2 Extraction as a Tool to Isolate Anti-Inflammatory Sesquiterpene Lactones from Cichorium intybus L. Roots" Molecules 26, no. 9: 2583. https://doi.org/10.3390/molecules26092583
APA StyleBaixinho, J. P., Anastácio, J. D., Ivasiv, V., Cankar, K., Bosch, D., Menezes, R., de Roode, M., dos Santos, C. N., Matias, A. A., & Fernández, N. (2021). Supercritical CO2 Extraction as a Tool to Isolate Anti-Inflammatory Sesquiterpene Lactones from Cichorium intybus L. Roots. Molecules, 26(9), 2583. https://doi.org/10.3390/molecules26092583