Chemical Identification of Specialized Metabolites from Sulla (Hedysarum coronarium L.) Collected in Southern Italy
Abstract
:1. Introduction
2. Results and Discussion
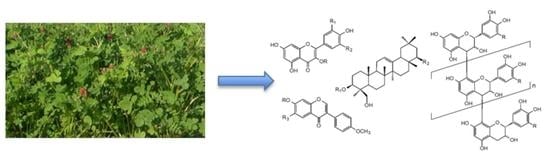
2.1. Identification and Quantitation of Flavonoids
2.2. Identification and Quantitation of Saponins
2.3. Identification and Quantitation of Proanthocyanidins
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Purification of Flavonoids
3.3. Extraction and Purification of Saponins
3.4. LC-ESI-MS Analysis and Identification of Flavonoids and Saponins
3.5. HPLC/DAD Analysis and Quantitation of Flavonoids and Saponins
3.6. Quantitation of Proanthocyanidins by the Butanol/HCl Assay
3.7. Extraction and Purification of Proanthocyanidins
3.8. Thiolysis of Proanthocyanidins
3.9. NMR Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, Y.; Zhao, Y.; Chen, H.; Wang, B.; Zhang, Q. Structure elucidation and complete NMR spectral assignment of two triterpenoid saponins from Radix Hedysari. Fitoterapia 2009, 80, 127–129. [Google Scholar] [CrossRef]
- Yang, M.; Sun, J.; Lu, Z.; Chen, G.; Guan, S.; Jiang, B.; Ye, M.; Guo, D. Phytochemical analysis of traditional Chinese medicine using liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2009, 1216, 2045–2062. [Google Scholar] [CrossRef]
- Dong, Y.; Tang, D.; Zhang, N.; Li, Y.; Zhang, C.; Li, L.; Li, M. Phytochemicals and biological studies of plants in genus Hedysarum. Chem. Cent. J. 2013, 7, 124. [Google Scholar] [CrossRef] [Green Version]
- Pires, T.C.S.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers: Emerging components in the diet. Trends Food. Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Burlando, B.; Pastorino, G.; Salis, A.; Damonte, G.; Clericuzio, M.; Cornara, L. The bioactivity of Hedysarum coronarium extracts on skin enzymes and cell correlates with phenolic content. Pharm. Biol. 2017, 55, 1984–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piano, E.; Pecetti, L. Minor legume species. In Fodder Crops and Amenity Grasses; Boller, B., Posselt, U.K., Veronesi, F., Eds.; Handbook of Plant Breeding, Series; Springer: New York, NY, USA, 2010; Volume 5, pp. 477–500. [Google Scholar]
- Annicchiarico, P.; Ruisi, P.; Di Miceli, G.; Pecetti, L. Morpho-physiological and adaptative variation of Italian germplasm of sulla (Hedysarum coronarium L.). Crop Pasture Sci. 2014, 65, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Barry, N.T.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the ruminant fed fresh temperate forage: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2013, 69, 32–48. [Google Scholar] [CrossRef]
- Sulas, L.; Campesi, G.; Piluzza, G.; Re, G.A.; Deligios, P.A.; Ledda, L.; Canu, S. Inoculation and N fertilization affect the dry matter, N fixation and bioactive compounds in sulla leaves. Agronomy 2019, 9, 289. [Google Scholar] [CrossRef] [Green Version]
- Barry, T.N.; McNabb, W.C. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Niezen, J.H.; Charleston, W.A.G.; Robertson, H.A.; Shelton, D.; Waghorn, G.C.; Green, R. The effect of feeding sulla (Hedysarum coronarium) or lucerne (Medicago sativa) on lamb parasite burdens and development of immunity to gastrointestinal nematodes. Vet. Parasitol. 2002, 105, 229–245. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Jonker, A.; Yu, P. The occurrence, biosynthesis, and molecular structure of proanthocyanidins and their effects on legume forage protein precipitation, digestion and absorption in the ruminant digestive tract. Int. J. Mol. Sci. 2017, 18, 1105. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüsker, A.; Niderkorn, V.; Pellikaan, W.; Salminen, J.-P.; et al. Benefit of condensed tannins in forage legumes fed to ruminants: Importance of structure, concentration, and diet composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, H.; Liu, Y.; Wang, W.; Zhao, Y.; Chen, H.; Liang, H.; Zhang, Q. Chemotaxonomy studies on the genus Hedysarum. Biochem. Syst. Ecol. 2019, 86, 103902. [Google Scholar] [CrossRef]
- Bai, H.Y.; Zheng, W.H.; Han, S.; Bao, F.; Sun, L.L.; Zhang, K.X.; Wang, L.Y.; Du, H.; Li, Y.M.; Feng, S.L.; et al. Metabolomic determination of specialized metabolites using chromatography-tandem mass spectrometry in the traditional Chinese medicines Astragali radix and Hedysari radix. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Neretina, O.V.; Fedorov, S.V.; Gromova, A.S.; Lutskii, V.L.; El’kin, Y.N. Flavonoids from Hedysarum setigerum. Chem. Nat. Compd. 2002, 38, 194–195. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Chen, H.; Wang, B.; An, D.; Zhao, Y. Structural determination of saponins from Hedysarum polybotrys. Magn. Reson. Chem. 2006, 44, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, W.; Sun, J.; Zhao, Y.; Liu, Y.; Liang, H.; Guo, D. Characterization of phenolic compounds in the crude extract of Hedysarum multijugum by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3833–3841. [Google Scholar] [CrossRef]
- Tibe, O.; Maegher, L.P.; Fraser, K.; Harding, D.R.K. Condensed tannins and flavonoids from the forage legume sulla (Hedysarum coronarium). J. Agric. Food Chem. 2011, 59, 9402–9409. [Google Scholar] [CrossRef]
- Ben Salah, N.; Casabianca, H.; Essaidi, I.; Chenavas, S.; Fildier, A.; Sanglar, C.; Ben Jannet, H.; Bouzouita, N. Isolation and structure elucidation of two new antioxidant flavonoid glycosides and fatty acids composition in Hedysarum carnosum Desf. Ind. Crops Prod. 2016, 81, 195–201. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.Y.; Chen, H.B.; Zhang, Q.Y.; Liang, H. Two new prenylated isoflavones from Hedysarum multijugum. J. Asian Nat. Prod. Res. 2017, 19, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Y.Y.; Wang, B.; Liang, H.; Tu, G.Z.; Chen, H.B. Two new arylbenzofurans from the roots of Hedysarum multijugum. J. Asian Nat. Prod. Res. 2007, 9, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Y.Y.; Liang, H.; Jia, Q.; Chen, H.B. Coumestans from Hedysarum multijugum. J. Nat. Prod. 2006, 69, 876–880. [Google Scholar] [CrossRef]
- Cheng, F.; Zou, K.; Zhu, S.; Katsuko, K. A new phenylpropionate derivative from the rhizomes of Hedysarum polybotris. Chin. J. Nat. Med. 2009, 7, 351–353. [Google Scholar] [CrossRef]
- Vukics, V.; Guttman, A. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2010, 29, 1–16. [Google Scholar] [CrossRef]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, Ł. Structural characterization of flavonoid glycoconjugates and their derivatives with mass spectrometric techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef] [Green Version]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Liu, Y.; Chen, H.B.; Zhao, Y.Y.; Wang, B.; Zhang, Q.Y.; Zhang, L.; Tu, P.F. Quantification and stability studies on the flavonoids of Radix hedysari. J. Agric. Food Chem. 2006, 54, 6634–6639. [Google Scholar] [CrossRef] [PubMed]
- Kudou, S.; Tonomura, M.; Tsukamoto, C.; Uchida, T.; Sakabe, T.; Tamura, N.; Okubo, K. Isolation and Structural Elucidation of DDMP-Conjugated Soyasaponins as Genuine Saponins from Soybean Seeds. Biosci. Biotech. Biochem. 1993, 57, 546–550. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, B.W.; Park, K.H.; Jeong, S.H.; Kim, H.T.; Ko, J.M.; Baek, I.Y.; Lee, J.H. Rapid characterisation and comparison of saponin profiles in the seeds of Korean Leguminous species using ultra performance liquid chromatography with photodiode array detector and electrospray ionisation/mass spectrometry (UPLC–PDA–ESI/MS) analysis. Food Chem. 2014, 146, 270–277. [Google Scholar] [CrossRef]
- Bianco, G.; Pascale, R.; Carbone, C.F.; Acquaviva, M.A.; Cataldi, T.R.I.; Schmitt-Kopplin, P.; Buchicchio, A.; Russo, D.; Milella, L. Determination of soyasaponins in Fagioli di Sarconi beans (Phaseolus vulgaris L.) by LC-ESI-FTICR-MS and evaluation of their hypoglycemic activity. Anal. Bioanal. Chem. 2018, 410, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, B.; Wang, S.R.; Kim, K.; Lee, J.H. Rapid characterization of metabolites in soybean using ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS/MS) and screening for a-glucosidase inhibitory and antioxidant properties through different solvent systems. J. Food Drug Anal. 2018, 26, 277–291. [Google Scholar]
- Tava, A.; Biazzi, E.; Ronga, D.; Mella, M.; Doria, F.; Accogli, R.; Argentieri, M.P.; Avato, P. Triterpenic saponins from Medicago marina L. Phytochemistry 2020, 174, 112333. [Google Scholar] [CrossRef] [PubMed]
- Niezen, J.H.; Waghorn, T.S.; Charleston, W.A.G.; Waghorn, G.C. Grown and gastrointestinal nematode parasitism in lambs grazing either Lucerne (Medicago sativa) or sulla (Hedysarum coronarium) which contains condensed tannins. J. Agric. Sci. 1995, 81, 102–109. [Google Scholar]
- Piluzza, G.; Bullitta, S.; Deroma, M.; Odoardi, M. The accumulation of condensed tannins in local populations of sulla. Cah. Options Mediterr. 2000, 45, 199–202. [Google Scholar]
- Gu, L.; Kelm, M.; Hammerstone, J.; Beecher, G.; Cunningham, D.; Vannozzi, S.; Prior, R. Fractionation of polymeric procyanidins from lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC MS fluorescent detection method. J. Agric. Food Chem. 2002, 50, 4852–4860. [Google Scholar] [CrossRef] [PubMed]
- Maegher, L.P.; Widdup, K.; Sivakumaran, S.; Lucas, R.; Rumball, W. Floral Trifolium proanthocyanidins: Polyphenol formation and compositional diversity. J. Agric. Food Chem. 2006, 54, 5482–5488. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, S.; Maegher, L.P.; Foo, L.Y.; Lane, G.A.; Fraser, K.; Rumball, W. Floral procyanidins of the forage legume red clover (Trifolium pratense L.). J. Agric. Food Chem. 2004, 52, 1581–1585. [Google Scholar] [CrossRef]
- Mattews, S.; Mila, I.; Scalbert, A.; Pollet, B.; Lapierre, C.; Hervé du Penhoat, C.L.M.; Rolando, C.; Donnelly, D.M.X. Method for estimation of proanthocyanidins based on their acid depolymerization in the presence of nucleophiles. J. Agric. Food Chem. 1997, 45, 1195–1201. [Google Scholar] [CrossRef]
- Czochanska, Z.; Foo, L.Y.; Newman, R.H.; Porter, L.J. Polymeric proanthocyanidins. Stereochemistry, structural units and molecular weight. J. Chem. Soc. Perkin Trans. I 1980, 2278–2286. [Google Scholar] [CrossRef]
- Porter, L.J.; Newmann, R.H.; Foo, L.Y.; Wong, H. Polymeric proanthocyanidins. 13C NMR studies of procyanidins. J. Chem. Soc. Perkin Trans. I 1982, 1, 1217–1221. [Google Scholar] [CrossRef]
- Thompson, D.; Pizzi, A. Simple 13C-NMR methods for quantitative determinations of polyflavonoid tannin characteristics. J. Appl. Polym. Sci. 1995, 55, 107–112. [Google Scholar] [CrossRef]
- Yang, H.; Kim, H.W.; Kwon, Y.S.; Kim, H.K.; Sung, S.H. Fast and simple discriminative analysis of anthocyanins-containing berries using LC/MS spectral data. Phytochem. Anal. 2017, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Pu, Y.; Yoo, C.G.; Ragauskas, A.J. The occurrence of tricin and its derivatives in plants. Green Chem. 2016, 18, 1439–1454. [Google Scholar] [CrossRef]
- Tava, A.; Avato, P. Chemical and biological activity of triterpene saponins from Medicago species. Nat. Prod. Commun. 2006, 1, 1159–1180. [Google Scholar] [CrossRef]
- Faizal, A.; Geelen, D. Saponins and their role in biological processes in plants. Phytochem. Rev. 2013, 12, 877–893. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Skrajda-Brdak, M.; Dabrowsky, G.; Konopka, I. Edible flowers, a source of valuable phytonutrients and their pro-healthy effects—A review. Trends Food Sci. Technol. 2020, 103, 179–199. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food source, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [Green Version]
- D’Addabbo, T.; Carbonara, T.; Leonetti, P.; Radicci, V.; Tava, A.; Avato, P. Control of plant parasitic nematodes with active saponins and biomass from Medicago sativa. Phytochem. Rev. 2011, 10, 503–519. [Google Scholar] [CrossRef]
- Avato, P.; Migoni, D.; Argentieri, M.; Fanizzi, F.P.; Tava, A. Activity of saponins from Medicago species against HeLa and MCF-7 cell lines and their capacity to potentiate cisplatin effect. Anti Cancer Agents Med. Chem. 2017, 17, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 15, 107–112. [Google Scholar] [CrossRef] [PubMed]



| # | UV lmax(nm) | [M + H]+ (m/z) | [M − H]− (m/z) | MW | Compound | Flowers | Leaves |
|---|---|---|---|---|---|---|---|
| 1 | 255, 270sh, 358 | 627, 481, 319 | 625, 607, 317 | 626 | Myricetin-3-O-rutinoside | 0.11 ± 0.04 | 0.02 ± 0.01 |
| 2 | 254, 266sh, 300sh, 354 | 757, 611, 465, 449, 303 | 755, 737, 609, 591, 489, 301 | 756 | Quercetin-3-O-(2″,6″-di-O-rhamnosyl)-glucoside | 1.60 ± 0.38 | 0.13 ± 0.03 |
| 3 | 265, 299sh, 325sh, 354 | 741, 595, 449, 433, 287 | 739, 721, 593, 575, 393, 285 | 740 | Kaempferol-3-O-(2″,6″-di-O-rhamnosyl)-glucoside | 1.15 ± 0.12 | - |
| 4 | 256, 267sh, 354 | 611, 465, 303 | 609, 591, 301 | 610 | Quercetin-3-O-rutinoside isomer | 0.27 ± 0.05 | 0.03 ± 0.02 |
| 5 | 265, 298sh, 353 | 741, 595, 449, 433, 287 | 739, 593, 575, 285 | 740 | Kaempferol-3-O-rutinoside-7-O-rhamnoside | 0.33 ± 0.03 | - |
| 6 | 256, 266sh, 299sh, 356 | 611, 465, 303 | 609, 591, 301 | 610 | Quercetin-3-O-rutinoside (rutin) | 1.15 ± 0.23 | 0.71 ± 0.07 |
| 7 | 255, 260sh, 355 | - | 491, 329 | 492 | Tricin-7-O-galactoside | - | 0.05 ± 0.02 |
| 8 | 255, 266sh, 354 | 771, 625, 479, 463, 317 | 769, 623, 605, 315 | 770 | Isorhamnetin-3-O-(2″,6″-di-O-rhamnosyl)-glucoside | 0.12 ± 0.02 | - |
| 9 | 263, 299sh, 354 | 595, 449, 287 | 593, 285 | 594 | Kaempferol-3-O-rutinoside (nicotiflorin) | 0.26 ± 0.02 | 0.25 ± 0.04 |
| 10 | 265, 299sh, 353 | - | 447, 285 | 448 | Kaempferol-3-O-galactoside | 0.02 ± 0.01 | - |
| 11 | 256, 267sh, 354 | 551, 533, 465, 287 | - | 550 | Quercetin-3-O-malonyl glucoside | - | 0.06 ± 0.02 |
| 12 | 256, 267sh, 355 | - | 505, 463, 301 | 506 | Quercetin-3-O-acetyl glucoside | - | 0.04 ± 0.01 |
| 13 | 264, 300sh, 355 | 595, 449, 287 | 593, 285- | 594 | Kaempferol-3-O-rutinoside isomer | 0.24 ± 0.04 | 0.14 ± 0.04 |
| 14 | 255, 266sh, 355 | 625, 479, 317 | 623, 315 | 624 | Isorhamnetin-3-O-rutinoside | 0.18 ± 0.01 | 0.38 ± 0.06 |
| 15 | 255, 266sh, 356 | 625, 479, 317 | 623, 315 | 624 | Isorhamnetin-3-O-rutinoside isomer | 0.05 ± 0.01 | 0.12 ± 0.06 |
| 16 | 254, 298sh, 357 | - | 655, 347 | 654 | 5,7-dihydroxy-3′,4′,5′-trimethoxyflavone-7-O-rutinoside | - | 0.08 ± 0.03 |
| 17 | 243, 287, 318 | - | 517, 337, 179 | 518 | Unidentified | 0.21 ± 0.03 | - |
| 18 | 253, 355 | - | 507, 417, 387, 345 | 508 | Unidentified | - | 0.06 ± 0.03 |
| 19 | 258, 329 | - | 475, 267 | 476 | Unidentified | - | 0.06 ± 0.02 |
| 20 | 259, 355sh | 431, 269 | - | 430 | Formononetin-7-O-glucoside (ononin) | 0.15 ± 0.06 | 0.22 ± 0.08 |
| 21 | 260, 320sh | 461, 299 | - | 460 | Afrormosin-7-O-glucoside | 0.04 ± 0.02 | - |
| 22 | 260, 355sh | 517, 431, 269 | - | 516 | Formononetin-7-O-glucoside-6″-O-malonate | 0.38 ± 0.06 | 0.37 ± 0.02 |
| 23 | 260, 320sh | 547, 461, 299 | - | 546 | Afrormosin-7-O-glucoside-6″-O-malonate | 0.02 ± 0.00 | - |
| Total | 6.26 ± 0.75 | 2.72 ± 0.38 |
| # | MW | Compound | Flowers | Leaves | |
|---|---|---|---|---|---|
| 24 | C48H78O18 | 942 | RhaGalGluA-SoyaB | 3.79 ± 0.25 | 1.63 ± 0.17 |
| 25 | C42H68O14 | 796 | GalGluA-SoyaB | 0.16 ± 0.02 | 0.02 ± 0.01 |
| 26 | C48H76O18 | 940 | RhaGalGluA-SoyaE | 0.10 ± 0.02 | 0.14 ± 0.02 |
| 27 | C42H66O14 | 794 | GalGluA-SoyaE | 0.41 ± 0.05 | 0.06 ± 0.01 |
| 28 | C54H84O21 | 1068 | RhaGalGluA-SoyaB-DDMP | 1.74 ± 0.30 | 1.02 ± 0.06 |
| 29 | C48H74O17 | 922 | GalGluA-SoyaB-DDMP | 0.09 ± 0.06 | 0.02 ± 0.01 |
| Total | 6.28 ± 0.30 | 2.88 ± 0.23 |
| % Terminal Units | % Extender Units | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mDP | C | EC | GC | EGC | C | EC | GC | EGC | PD:PC | |
| Total | 21 | 57 | 7 | 30 | 5 | 5 | 7 | 32 | 56 | 88:12 |
| Fraction 1 | 15 | 76 | 3 | 20 | 1 | 7 | 8 | 38 | 47 | 85:15 |
| Fraction 2 | 25 | 51 | 19 | 19 | 11 | 4 | 7 | 33 | 57 | 90:10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tava, A.; Biazzi, E.; Ronga, D.; Mella, M.; Doria, F.; D’Addabbo, T.; Candido, V.; Avato, P. Chemical Identification of Specialized Metabolites from Sulla (Hedysarum coronarium L.) Collected in Southern Italy. Molecules 2021, 26, 4606. https://doi.org/10.3390/molecules26154606
Tava A, Biazzi E, Ronga D, Mella M, Doria F, D’Addabbo T, Candido V, Avato P. Chemical Identification of Specialized Metabolites from Sulla (Hedysarum coronarium L.) Collected in Southern Italy. Molecules. 2021; 26(15):4606. https://doi.org/10.3390/molecules26154606
Chicago/Turabian StyleTava, Aldo, Elisa Biazzi, Domenico Ronga, Mariella Mella, Filippo Doria, Trifone D’Addabbo, Vincenzo Candido, and Pinarosa Avato. 2021. "Chemical Identification of Specialized Metabolites from Sulla (Hedysarum coronarium L.) Collected in Southern Italy" Molecules 26, no. 15: 4606. https://doi.org/10.3390/molecules26154606
APA StyleTava, A., Biazzi, E., Ronga, D., Mella, M., Doria, F., D’Addabbo, T., Candido, V., & Avato, P. (2021). Chemical Identification of Specialized Metabolites from Sulla (Hedysarum coronarium L.) Collected in Southern Italy. Molecules, 26(15), 4606. https://doi.org/10.3390/molecules26154606