Curcumin and Resveratrol Improve Muscle Function and Structure through Attenuation of Proteolytic Markers in Experimental Cancer-Induced Cachexia
Abstract
:1. Introduction
2. Methods
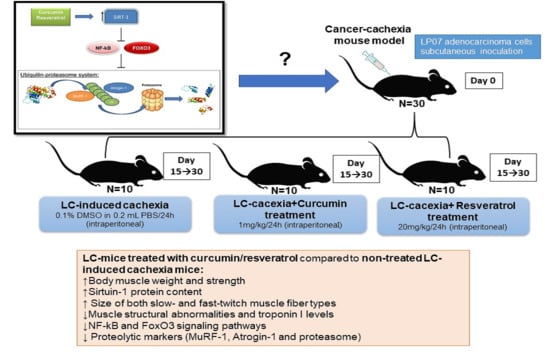
2.1. Animal Experiments
2.1.1. Tumor Inoculation and Treatments
2.1.2. Experimental Protocol
2.1.3. Studies in Mice: In Vivo Measurements
2.1.4. Sacrifice and Sample Collection
2.2. Biological Analyses
2.2.1. Immunoblotting of 1D Electrophoresis
2.2.2. Enzyme-Linked ImmunoSorbent Assay (ELISA) Plasma Skeletal Muscle Troponin-I Levels
2.2.3. Muscle Fiber Typing and Morphometry
2.2.4. Muscle Morphological Features
2.2.5. Statistical Analysis
3. Results
3.1. Physiological Characteristics of the Study Animals
3.2. Structural Phenotypic Characteristics
3.3. Sirtuin-1 Protein Content
3.4. Muscle Specific Proteins
3.5. Muscle Proteolytic Markers
3.6. Muscle Atrophy Signaling Markers
4. Discussion
Study Critique
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Argilés, J.M.; Busquets, S.; López-Soriano, F.J. Cancer cachexia, a clinical challenge. Curr. Opin. Oncol. 2019, 31, 286–290. [Google Scholar] [CrossRef]
- Barreiro, E.; Sznajder, J.I.; Nader, G.A.; Budinger, G.R.S. Muscle dysfunction in patients with lung diseases a growing epidemic. Am. J. Respir. Crit. Care Med. 2015, 191, 616–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacon-Cabrera, A.; Fermoselle, C.; Urtreger, A.J.; Mateu-Jimenez, M.; Diament, M.J.; de Kier Joffé, E.D.B.; Sandri, M.; Barreiro, E. Pharmacological Strategies in Lung Cancer-Induced Cachexia: Effects on Muscle Proteolysis, Autophagy, Structure, and Weakness. J. Cell. Physiol. 2014, 229, 1660–1672. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Degracia, A.; Blanco, D.; Vilà-Ubach, M.; Biurrun, G.; Solórzano, C.O.; Montuenga, L.M.; Barreiro, E. Phenotypic and metabolic features of mouse diaphragm and gastrocnemius muscles in chronic lung carcinogenesis: Influence of underlying emphysema. J. Transl. Med. 2016, 14, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-Degracia, A.; Busquets, S.; Argilés, J.M.M.; Bargalló-Gispert, N.; López-Soriano, F.J.J.; Barreiro, E. Effects of the beta 2 agonist formoterol on atrophy signaling, autophagy, and muscle phenotype in respiratory and limb muscles of rats with cancer-induced cachexia. Biochimie 2018, 149, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-Degracia, A.; Busquets, S.; Argilés, J.M.; López-Soriano, F.J.; Barreiro, E. Formoterol attenuates increased oxidative stress and myosin protein loss in respiratory and limb muscles of cancer cachectic rats. PeerJ 2017, 5, e4109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-Degracia, A.; Granado-Martínez, P.; Millán-Sánchez, A.; Tang, J.; Pons-Carreto, A.; Barreiro, E. Reduced lung cancer burden by selective immunomodulators elicits improvements in muscle proteolysis and strength in cachectic mice. J. Cell. Physiol. 2019, 234, 18041–18052. [Google Scholar] [CrossRef]
- Puig-Vilanova, E.; Rodriguez, D.A.; Lloreta, J.; Ausin, P.; Pascual-Guardia, S.; Broquetas, J.; Roca, J.; Gea, J.; Barreiro, E. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic. Biol. Med. 2015, 79, 91–108. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Commentary Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Sheng, X.; Zhang, X.; Guo, M.; Ji, X. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des. Devel. Ther. 2016, 10, 1267–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, W.; Yang, Y.; Yan, J.; Yu, S.; Liu, J.; Zhou, J.; Zhang, J.; Jin, Z.; Yi, D. The effects of curcumin post-treatment against myocardial ischemia and reperfusion by activation of the JAK2/STAT3 signaling pathway. Basic Res. Cardiol. 2012, 107, 263. [Google Scholar] [CrossRef]
- Receno, C.N.; Liang, C.; Korol, D.L.; Atalay, M.; Heffernan, K.S.; Brutsaert, T.D.; Deruisseau, K.C. Effects of prolonged dietary curcumin exposure on skeletal muscle biochemical and functional responses of aged male rats. Int. J. Mol. Sci. 2019, 20, 1178. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Ye, B.; Dai, Z.; Wu, X.; Lu, Z.; Shan, P.; Huang, W. Curcumin inhibits autophagy and apoptosis in hypoxia/reoxygenation-induced myocytes. Mol. Med. Rep. 2015, 11, 4678–4684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaloor, D.; Miller, K.J.; Gephart, J.; Mitchell, P.O.; Pavlath, G.K. Systemic administration of the NF-κB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am. J. Physiol. Cell Physiol. 1999, 277. [Google Scholar] [CrossRef] [PubMed]
- Mañas-García, L.; Guitart, M.; Duran, X.; Barreiro, E. Satellite cells and markers of muscle regeneration during unloading and reloading: Effects of treatment with resveratrol and curcumin. Nutrients 2020, 12, 1870. [Google Scholar] [CrossRef] [PubMed]
- Busquets, S.; Carbó, N.; Almendro, V.; Quiles, M.T.; López-Soriano, F.J.; Argilés, J.M. Curcumin, a natural product present in turmeric, decreases tumor growth but does not behave as an anticachectic compound in a rat model. Cancer Lett. 2001, 167, 33–38. [Google Scholar] [CrossRef]
- Jackson, J.R.; Ryan, M.J.; Hao, Y.; Alway, S.E. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1572–R1581. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Carpéné, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.K.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Chen, S.; Li, Z.; Zhao, X.; Li, W.; Sun, Y.; Zhang, Z.; Ling, W.; Feng, X. Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice. Nutr. Metab. 2014, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Cheng, X.; Cui, Y.; Xia, Q.; Yan, X.; Zhang, M.; Lan, G.; Liu, J.; Shan, T.; Huang, Y. Resveratrol regulates skeletal muscle fibers switching through the AdipoR1-AMPK-PGC-1α pathway. Food Funct. 2019, 10, 3334–3343. [Google Scholar] [CrossRef] [Green Version]
- Menzies, K.J.; Singh, K.; Saleem, A.; Hood, D.A. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J. Biol. Chem. 2013, 288, 6968–6979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarolim, S.; Millen, J.; Heeren, G.; Laun, P.; Goldfarb, D.S.; Breitenbach, M. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004, 5, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; He, Z.; Mao, C.; Shui, X.; Cai, L. Therapeutic Effects of Resveratrol Liposome on Muscle Injury in Rats. Med. Sci. Monit. 2019, 25, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Busquets, S.; Fuster, G.; Ametller, E.; Olivan, M.; Figueras, M.; Costelli, P.; Carbó, N.; Argilés, J.M.; López-Soriano, F.J. Resveratrol does not ameliorate muscle wasting in different types of cancer cachexia models. Clin. Nutr. 2007, 26, 239–244. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Abd El Mohsen, M.M.; Minihane, A.-M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mañas-garcía, L.; Bargalló, N.; Gea, J.; Barreiro, E. Muscle phenotype, proteolysis, and atrophy signaling during reloading in mice: Effects of curcumin on the gastrocnemius. Nutrients 2020, 12, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mañas-García, L.; Penedo-Vázquez, A.; López-Postigo, A.; Deschrevel, J.; Durán, X.; Barreiro, E. Prolonged immobilization exacerbates the loss of muscle mass and function induced by cancer-associated cachexia through enhanced proteolysis in mice. Int. J. Mol. Sci. 2020, 21, 8167. [Google Scholar] [CrossRef]
- Chacon-Cabrera, A.; Fermoselle, C.; Salmela, I.; Yelamos, J.; Barreiro, E. MicroRNA expression and protein acetylation pattern in respiratory and limb muscles of Parp-1-/- and Parp-2-/- mice with lung cancer cachexia. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2530–2543. [Google Scholar] [CrossRef] [Green Version]
- Urtreger, A.J.; Diament, M.J.; Ranuncolo, S.M.; Del, C. Vidal, M.; Puricelli, L.I.; Klein, S.M.; De Kier Joffe, E.D. New murine cell line derived from a spontaneous lung tumor induces paraneoplastic syndromes. Int. J. Oncol. 2001, 18, 639–647. [Google Scholar] [CrossRef]
- Chacon-Cabrera, A.; Mateu-Jimenez, M.; Langohr, K.; Fermoselle, C.; García-Arumí, E.; Andreu, A.L.; Yelamos, J.; Barreiro, E. Role of PARP activity in lung cancer-induced cachexia: Effects on muscle oxidative stress, proteolysis, anabolic markers, and phenotype. J. Cell. Physiol. 2017, 232, 3744–3761. [Google Scholar] [CrossRef]
- Pardo, P.S.; Boriek, A.M. The physiological roles of Sirt1 in skeletal muscle. Aging 2011, 3, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Ryall, J.G.; Dell’Orso, S.; Derfoul, A.; Juan, A.; Zare, H.; Feng, X.; Clermont, D.; Koulnis, M.; Gutierrez-Cruz, G.; Fulco, M.; et al. The NAD+-dependent sirt1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 2015, 16, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreiro, E.; Puig-Vilanova, E.; Salazar-Degracia, A.; Pascual-Guardia, S.; Casadevall, C.; Gea, J. The phosphodiesterase-4 inhibitor roflumilast reverts proteolysis in skeletal muscle cells of patients with COPD cachexia. J. Appl. Physiol. 2018, 125, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Murillo Ortiz, B.O.; Fuentes Preciado, A.R.; Ramírez Emiliano, J.; Martínez Garza, S.; Ramos Rodríguez, E.; de Alba Macías, L.A. Recovery of Bone and Muscle Mass in Patients with Chronic Kidney Disease and Iron Overload on Hemodialysis and Taking Combined Supplementation with Curcumin and Resveratrol. Clin. Interv. Aging 2019, 14, 2055–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.; Wang, W.; He, G.; Kuick, R.D.; Gossner, G.; Kueck, A.S.; Wahl, H.; Opipari, A.W.; Liu, J.R. Resveratrol inhibits ovarian tumor growth in an in vivo mouse model. Cancer 2016, 122, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Maiuri, M.C.; Markaki, M.; Megalou, E.; Pasparaki, A.; Palikaras, K.; Criollo, A.; Galluzzi, L.; Malik, S.A.; Vitale, I.; et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010, 1, e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. Ser. A 2017, 72, 1595–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and skeletal muscle mass: The role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 2015, 14, 511–523. [Google Scholar] [CrossRef]
- Jhanji, M.; Rao, C.N.; Sajish, M. Towards resolving the enigma of the dichotomy of resveratrol: Cis- and trans-resveratrol have opposite effects on TyrRS-regulated PARP1 activation. GeroScience 2021, 43, 1171–1200. [Google Scholar] [CrossRef]
- Sajish, M.; Schimmel, P. A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature 2015, 519, 370–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Castillejo, S.; Macià, A.; Motilva, M.J.; Catalán, Ú.; Solà, R. Endothelial Cells Deconjugate Resveratrol Metabolites to Free Resveratrol: A Possible Role in Tissue Factor Modulation. Mol. Nutr. Food Res. 2019, 63, 1800715. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Jhanji, M.; Murphy, K.; Gower, R.M.; Sajish, M.; Jabbarzadeh, E. Nicotinamide Augments the Anti-Inflammatory Properties of Resveratrol through PARP1 Activation. Sci. Rep. 2019, 9, 10219. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| LC-Induced Cachexia (N = 10) | LC-Cachexia + Curcumin (N = 10) | LC-Cachexia + Resveratrol (N = 10) | |
|---|---|---|---|
| Age at baseline (weeks) | 10 | 10 | 10 |
| Body weight at baseline (g) | 20.43 (1.08) | 20.31 (1.13) | 20.3 (1.08) |
| Final body weight (g) | 15.76 (1.67) | 18.5 (1.31) *** | 19.25 (1.55) *** |
| Body weight gain (%) | −22.87 (6.98) | −8.87 (4.35) *** | −5.22 (3.8) *** |
| Food intake (g/24 h) | 2.53 (0.52) | 2.64 (0.58) | 2.69 (0.58) |
| Gastrocnemius weight (g) | 0.086 (0.01) | 0.105 (0.01) *** | 0.104 (0.008) *** |
| Soleus weight (g) | 0.0064 (0.001) | 0.0075 (0.001) * | 0.0073 (0.001) * |
| Tumor weight (g) | 2.136 (0.56) | 1.77 (0.41) (17%) | 1.49 (0.79) * (30%) |
| Limb strength gain (%) | −25.01 (2.29) | −1.51 (3.29) ***, +93% | −5.27 (6.9) ***, +78% |
| Muscle | LC-Induced Cachexia (N = 10) | LC-Cachexia + Curcumin (N = 10) | LC-Cachexia + Resveratrol (N = 10) | |
|---|---|---|---|---|
| Muscle fiber type, % | ||||
| Type I fibers | Gastrocnemius | 12.34 (1.9) | 16.03 (4.4) | 14.16 (3.4) |
| Soleus | 49.08 (5.01) | 52.98 (9.38) | 54.87 (5.94) | |
| Type II fibers | Gastrocnemius | 87.65 (1.9) | 83.97 (4.4) | 85.83 (3.4) |
| Soleus | 50.92 (5.01) | 47.02 (9.38) | 45.13 (5.94) | |
| Cross-sectional areas | ||||
| Type I fibers (µm2) | Gastrocnemius | 499.34 (71.9) | 695.06 (136.63) *, +34% | 711.44 (240.26) ***, +36% |
| Soleus | 528.65 (111.06) | 799 (159.48) **, +51% | 770.66 (102.86) **, +46% | |
| Type II fibers (µm2) | Gastrocnemius | 464.79 (53.88) | 688.04 (132.61) ***, +49% | 648.2 (134.2) **, +34% |
| Soleus | 443.16 (69.50) | 603.17 (103.95) **, +36% | 592.01 (99.27) *, +34% | |
| Muscle structural abnormalities, % | ||||
| Total abnormal fraction | Gastrocnemius | 3.8 (0.84) | 2.85 (0.94) * | 1.9 (0.27) *** |
| Soleus | 9.25 (1.88) | 3.57 (0.55) *** | 3.73 (0.39) *** | |
| Internal nuclei | Gastrocnemius | 2.34 (0.54) | 2.23 (0.8) | 1.21 (0.2) ** |
| Soleus | 6.08 (1.36) | 3.17 (0.43) *** | 2.3 (0.5) *** | |
| Inflammatory cells | Gastrocnemius | 1.26 (0.59) | 0.5 (0.32) * | 0.58 (0.31) * |
| Soleus | 1.56 (1.48) | 0.36 (0.29) * | 0.49 (0.3) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penedo-Vázquez, A.; Duran, X.; Mateu, J.; López-Postigo, A.; Barreiro, E. Curcumin and Resveratrol Improve Muscle Function and Structure through Attenuation of Proteolytic Markers in Experimental Cancer-Induced Cachexia. Molecules 2021, 26, 4904. https://doi.org/10.3390/molecules26164904
Penedo-Vázquez A, Duran X, Mateu J, López-Postigo A, Barreiro E. Curcumin and Resveratrol Improve Muscle Function and Structure through Attenuation of Proteolytic Markers in Experimental Cancer-Induced Cachexia. Molecules. 2021; 26(16):4904. https://doi.org/10.3390/molecules26164904
Chicago/Turabian StylePenedo-Vázquez, Antonio, Xavier Duran, Javier Mateu, Adrián López-Postigo, and Esther Barreiro. 2021. "Curcumin and Resveratrol Improve Muscle Function and Structure through Attenuation of Proteolytic Markers in Experimental Cancer-Induced Cachexia" Molecules 26, no. 16: 4904. https://doi.org/10.3390/molecules26164904
APA StylePenedo-Vázquez, A., Duran, X., Mateu, J., López-Postigo, A., & Barreiro, E. (2021). Curcumin and Resveratrol Improve Muscle Function and Structure through Attenuation of Proteolytic Markers in Experimental Cancer-Induced Cachexia. Molecules, 26(16), 4904. https://doi.org/10.3390/molecules26164904