Modulation of the PGE2-Mediated Pathway in the Eclosion Blocking Effect of Flumethrin and Terpenoid Subfraction Isolated from Artemesia nilagirica in Rhipicephalus annulatus
Abstract
:1. Introduction
2. Results and Discussion
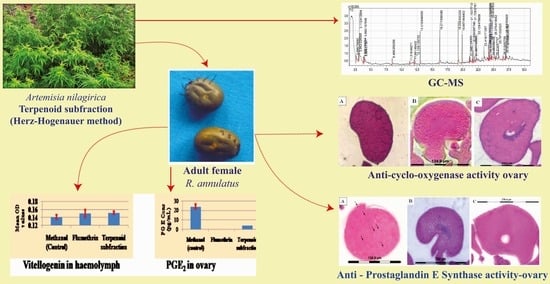
2.1. Gas Chromatography–Mass Spectrometry Analysis of Terpenoid Subfraction
2.2. Adult Immersion Test
2.3. Histology of Tick Ovary
2.3.1. Immunoperoxidase Staining of the Ovarian Sections with Anti-COX1 Antibodies
2.3.2. Immunoperoxidase Staining with Anti-PGES Antibodies of the Ovarian Sections
2.4. Quantification of PGE2 in the Engorged Tick Ovaries by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS-MS)
2.4.1. Response Linearity of PGE2
2.4.2. Detection and Recovery of PGE2 from Tick Ovaries
2.4.3. Detection of PGE2 in the Treated Tick Ovarian Samples
2.5. Quantification of Vitellogenin (Vg) in the Hemolymph of R. annulatus Tick Using Indirect ELISA
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.2.1. Preparation of Terpenoid Subfraction
3.2.2. Gas Chromatography–Mass Spectrometry Analysis of Terpenoid Subfraction
3.3. Ticks
Adult Immersion Test (AIT)
3.4. Modulation of Prostaglandin-Mediated Pathway
3.4.1. Calculation of Median Lethal Concentration (LC50)
3.4.2. Localization of Cyclooxygenase1 (COX1) and Prostaglandin E Synthase (PGES) Enzymes in the Tick Ovary
3.4.3. Determination of the Levels of PGE2 in Engorged Tick Ovaries by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS-MS)
Instrumentation
Sample Preparation
3.4.4. Indirect Enzyme-Linked Immunosorbent Assay (ELISA) for the Estimation of Vitellogenin Concentration in the Hemolymph of Tick
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Estrada-Peña, A.; de la Fuente, J.; Ostfeld, R.S.; Cabezas-Cruz, A. Interactions between tick and transmitted pathogens evolved to minimise competition through nested and coherent networks. Sci. Rep. 2015, 5, 10361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, T.D.; Shao, R.; Labruna, M.B.; Barker, S.C. Molecular phylogeny of soft ticks (Ixodida: Argasidae) inferred from mitochondrial genome and nuclear rRNA sequences. Ticks Tick Borne Dis. 2014, 5, 195–207. [Google Scholar] [CrossRef]
- Ghosh, S.; Azhahianambi, P.; Yadav, M.P. Upcoming and future strategies of tick control: A review. J. Vector Borne. Dis. 2007, 44, 79–89. [Google Scholar]
- Kumar, K.G.A.; Ravindran, R.; Johns, J.; Chandy, G.; Rajagopal, K.; Chandrasekhar, L.; George, A.J.; Ghosh, S. Ixodid tick vectors of wild mammals and reptiles of southern India. J. Arthropod Borne Dis. 2018, 12, 276–285. [Google Scholar]
- Nimisha, M.; Devassy, J.K.; Pradeep, R.K.; Pakideery, V.; Sruthi, M.K.; Pious, A.; Kurbet, P.S.; Amrutha, B.M.; Chandrasekhar, L.; Deepa, C.K.; et al. Ticks and accompanying pathogens of domestic and wild animals of Kerala, South India. Exp. Appl. Acarol. 2019, 79, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2005, 129, S3. [Google Scholar] [CrossRef]
- Guleria, S.; Tiku, A.K. Botanicals in Pest Management: Current Status and Future Perspectives. In Integrated Pest Management: Volume 1: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordecht, The Netherlands, 2009; p. 690. [Google Scholar]
- Ravindran, R.; Juliet, S.; Sunil, A.R.; Kumar, K.G.A.; Nair, S.N.; Amithamol, K.K.; Shynu, M.; Rawat, A.K.S.; Ghosh, S. Eclosion blocking effect of ethanolic extract of Leucas aspera (Lamiaceae) on Rhipicephalus (Boophilus) annulatus. Vet. Parasitol. 2011, 179, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Juliet, S.; Sunil, A.R.; Ajith Kumar, K.G.; Nair, S.N.; Amithamol, K.K.; Bandyopadhyay, A.; Rawat, A.K.S.; Ghosh, S. Acaricidal activity of Cassia alata against Rhipicephalus (Boophilus) annulatus. Exp. Appl. Acarol. 2012, 56, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Juliet, S.; Ravindran, R.; Sunil, A.R.; Kumar, K.G.A.; Nair, S.N.; Amithamol, K.K.; Bandyapadhyay, A.; Rawat, A.K.S.; Ghosh, S. Jatropha curcass (Linn) leaf extract–A possible alternative for population control of Rhipicephalus (Boophilus) annulatus. Asian Pac. J. Trop. Dis. 2012, 2, 225–229. [Google Scholar] [CrossRef]
- Sunil, A.R.; Amithamol, K.K.; Juliet, S.; Nair, S.N.; Kumar, K.G.A.; Soorya, V.C.; Divya, T.M.; Jyothimol, G.; Ghosh, S.; Ravindran, R. Acaricidal effect of Cassia fistula Linn. leaf ethanolic extract against Rhipicephalus (Boophilus) annulatus. Trop. Biomed. 2013, 30, 231–237. [Google Scholar]
- Divya, T.M.; Soorya, V.C.; Amithamol, K.K.; Juliet, S.; Ravindran, R.; Nair, S.N.; Ajithkumar, K.G. Acaricidal activity of alkaloid fractions of Leucas indica Spreng against Rhipicephalus (Boophilus) annulatus tick. Trop. Biomed. 2014, 31, 46–53. [Google Scholar]
- Krishna, T.P.A.; Krishna, T.P.A.; Chitra, N.D.; Deepa, P.E.; Udayan, D.; Sreelekha, K.P.; Juliet, S.; Nair, S.N.; Ravindran, R.; Ajithkumar, K.G.; et al. Acaricidal activity of petroleum ether extract of Tetrastigma leucostaphylum (Dennst.) Alston against Rhipicephalus (Boophilus) annulatus. Sci. World J. 2014, 2014, 715481. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, R.; Chithra, N.D.; Deepa, P.E.; Juliet, S.; Kumar, K.G.A.; Nair, S.N.; Udayan, D.; Nanjundappa, S.; Chandrasekhar, L.; Ghosh, S. Contrasting effects of ethanolic extracts of leaf and flower of Chromolaena odorata against Rhipicephalus (Boophilus) annulates. Ind. J. Ani. Sci. 2015, 85, 844–848. [Google Scholar]
- Palayullaparambil, A.K.T.; Palayullaparambil, A.K.T.; Juliet, S.; Renganathan, K.; Raju, R.; Athalathil, S.; Ravindran, R.; Chandrashekar, L.; Nair, S.N.; Ghosh, S. Pharmaco-chemical characterization and acaricidal activity of ethanolic extract of Chassalia curviflora (Wall ex Kurz.) Thwaites. Pharmacogn. J. 2016, 8, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, R.; Juliet, S.; Ramankutty, S.A.; Sathish, N.; Nair, S.N.; Ajithkumar, K.G.; Chandrasekhar, L.; Ghosh, S. Effects of ethanolic extract of the leaves of Pongamia glabra and Gliricidia sepium against Rhipicephalus (Boophilus) annulatus. Adv. Anim. Vet. Sci. 2017, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.N.; Juliet, S.; Amithamol, K.K.; Sunil, A.R.; Palayullaparambil, A.K.T.; Sreelekha, K.; Divya, T.M.; Udayan, D.; Kumar, K.G.A.; Ghosh, S.; et al. In vitro acaricidal effects of ethanolic extract and its fractions of Ageratum conyzoides L. against common cattle tick Rhipicephalus (Boophilus) annulatus. Ann. Phytomed. 2017, 6, 162–168. [Google Scholar] [CrossRef]
- Divya, T.M.; Soorya, V.C.; Amithamol, K.K.; Udayan, D.; Sreelekha, K.; Nair, S.N.; Ajithkumar, K.G.; Juliet, S.; Ravindran, R.; Ghosh, S. Acaricidal activity of crude ethanolic extract of Sphaeranthus indicus, its fractions and subfractions against Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 787–797. [Google Scholar] [CrossRef]
- Konig, I.F.M.; Oliveira, M.V.S.; Goncalves, R.R.P.; Peconick, A.P.; Thomasi, S.S.; Anholeto, L.A.; Lima-de-Souza, J.R.; Camargo-Mathias, M.I.; Remedio, R.N. Low concentrations of acetylcarvacrol induce drastic morphological damages in ovaries of surviving Rhipicephalus sanguineus sensu lato ticks (Acari: Ixodidae). Micron 2020, 129, 102780. [Google Scholar] [CrossRef] [PubMed]
- Nwanade, C.F.; Yu, Z.; Liu, J. Botanical acaricides induced morphophysiological changes of reproductive and salivary glands in tick: A mini-review. Res. Vet. Sci. 2020, 132, 285–291. [Google Scholar] [CrossRef]
- Denardi, S.E.; Bechara, G.H.; de Oliveira, P.R.; Camargo-Mathias, M.I. Azadirachta indica A. Juss (neem) induced morphological changes on oocytes of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) tick females. Exp. Parasitol. 2010, 126, 462–470. [Google Scholar] [CrossRef]
- Denardi, S.E.; Bechara, G.H.; de Oliveira, P.R.; Camargo-Mathias, M.I. Inhibitory action of neem aqueous extract (Azadirachta indica A. Juss) on the vitellogenesis of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks. Microsc. Res. Tech. 2011, 74, 889–899. [Google Scholar] [CrossRef]
- Arnosti, A.; Brienza, P.D.; Furquim, K.C.S.; Chierice, G.O.; Bechara, G.H.; Calligaris, I.B.; Camargo-Mathias, M.I. Effects of ricinoleic acid esters from castor oil of Ricinus communis on the vitellogenesis of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks. Exp. Parasitol. 2011, 127, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Denardi, S.E.; Bechara, G.H.; Oliveira, P.R.; Camargo-Mathias, M.I. Ultrastructural analysis of the oocytes of female Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks subjected to the action of Azadirachta indica A. Juss (neem). Ultrastruct. Pathol. 2012, 36, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Vendramini, M.C.R.; Camargo-Mathias, M.I.; de Faria, A.U.; Bechara, G.H.; de Oliveira, P.R.; Roma, G.C. Cytotoxic effects of andiroba oil (Carapa guianensis) in reproductive system of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) semiengorged females. Parasitol. Res. 2012, 111, 1885–1894. [Google Scholar] [CrossRef]
- Vendramini, M.C.R.; Camargo-Mathias, M.I.; De Faria, A.U.; Furquim, K.C.S.; De Souza, L.P.; Bechara, G.H.; Roma, G.C. Action of andiroba oil (Carapa guianensis) on Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) semi-engorged females: Morphophysiological evaluation of reproductive system. Microsc. Res. Tech. 2012, 75, 1745–1754. [Google Scholar] [CrossRef]
- Sampieri, B.R.; Arnosti, A.; Furquim, K.C.S.; Chierice, G.O.; Bechara, G.H.; de Carvalho, P.L.P.F.; Nunes, P.H.; Camargo-Mathias, M.I. Effect of ricinoleic acid esters from castor oil (Ricinus communis) on the oocyte yolk components of the tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Vet. Parasitol. 2013, 191, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, R.S.; Daemon, E.; Camargo-Mathias, M.I.; Furquim, K.C.S.; Sampieri, B.R.; Remédio, R.N.; Araújo, L.X.; Novato, T.P.L. Histopathological study of ovaries of Rhipicephalus sanguineus (Acari: Ixodidae) exposed to different thymol concentrations. Parasitol. Res. 2014, 113, 4555–4565. [Google Scholar] [CrossRef] [PubMed]
- Remedio, R.N.; Nunes, P.H.; Anholeto, L.A.; Oliveira, P.R.; Camargo-Mathias, M.I. Morphological effects of neem (Azadirachta indica A. Juss) seed oil with known azadirachtin concentrations on the oocytes of semi-engorged Rhipicephalus sanguineus ticks (Acari: Ixodidae). Parasitol. Res. 2015, 114, 431–444. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Anholeto, L.A.; Ferreira Rodrigues, R.A.; Arnosti, A.; Bechara, G.H.; de Carvalho Castro, K.N.; Camargo-Mathias, M.I. Cytotoxic effects of extract of Acmella oleracea in the ovaries and midgut of Rhipicephalus sanguineus Latreille, 1806 (Acari: Ixodidae) female ticks. J. Microsc. Ultrastruct. 2019, 7, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Konig, I.F.M.; Gonçalves, R.R.P.; Oliveira, M.V.S.; Silva, C.M.; Thomasi, S.S.; Peconick, A.P.; Remedio, R.N. Sublethal concentrations of acetylcarvacrol strongly impact oocyte development of engorged female cattle ticks Rhipicephalus microplus (Canestrini, 1888) (Acari: Ixodidae). Ticks Tick Borne Dis. 2019, 10, 766–774. [Google Scholar] [CrossRef]
- De Queiroz, V.T.; Campos, N.C.; Nunes, E.T.; Costa, A.V.; Coelho, J.D.; Trivilin, L.O.; de Melo, D.C.A.; Morais, P.A.B.; Martins, I.V.F. 1,8-cineole and castor oil in sodium lauryl ether sulphate disrupt reproduction and ovarian tissue of Rhipicephalus (Boophilus) microplus. Med. Vet. Entomol. 2020, 34, 316–326. [Google Scholar] [CrossRef]
- de Oliveira, P.R.; Gervásio, H.B.; Camargo-Mathias, M.I. Evaluation of cytotoxic effects of fipronil on ovaries of semi-engorged Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) tick female. Food Chem. Toxicol. 2008, 46, 2459–2465. [Google Scholar] [CrossRef]
- De Oliveira, P.R.; Gervásio, H.B.; Morales, M.A.M.; Camargo-Mathias, M.I.C. Action of the chemical agent fipronil on the reproductive process of semi-engorged females of the tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Ultrastructural evaluation of ovary cells. Food Chem. Toxicol. 2009, 47, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.P.M.; Oliveira, P.R.; Furquim, K.C.S.; Bechara, G.H.; Camargo-Mathias, M.I. Effects of fipronil (active ingredient of Frontline®) on salivary gland cells of Rhipicephalus sanguineus females (Latreille, 1806) (Acari: Ixodidae). Vet. Parasitol. 2009, 166, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Roma, G.C.; de Oliveira, P.R.; Pizano, M.A.; Mathias, M.I. Determination of LC50 of permethrin acaricide in semi-engorged females of the tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Exp. Parasitol. 2009, 123, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Roma, G.C.; Furquim, K.C.S.; Bechara, G.H.; Camargo-Mathias, M.I. Permethrin-induced morphological changes in oocytes of Rhipicephalus sanguineus (Acari: Ixodidae) semi-engorged females. Food Chem. Toxicol. 2010, 48, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Camargo-Mathias, M.I.; Pereira, N.R.C.; da Silva Reis, C.; de Almeida, C.R.; dos Santos Mendes, D.R.; de Araújo, G.B.; Postali, L.; Figueroa, T.; Ferreira, A.R.F.; Santos, J.P.; et al. Deltamethrin as inductor agent of precocious ovarian degeneration in Rhipicephalus sanguineus (Acari: Ixodidae) ticks. Exp. Appl. Acarol. 2017, 72, 161–169. [Google Scholar] [CrossRef]
- Kanapadinchareveetil, S.; Chandrasekhar, L.; Kartha, H.S.; Ravindran, R.; Juliet, S.; Ajithkumar, K.G.; Nair, S.N.; Ghosh, S. Ultrastructural analysis of oocytes of Rhipicephalus (Boophilus) annulatus during postengorgement period as a tool to evaluate the cytotoxic effects of amitraz and deltamethrin on the germinative cells. Vet. Parasitol. 2017, 247, 113–120. [Google Scholar] [CrossRef]
- Kanapadinchareveetil, S.; Chandrasekhar, L.; Pious, A.; Kartha, H.S.; Ravindran, R.; Juliet, S.; Nair, S.N.; Ajithkumar, K.G.; Ghosh, S. Molecular, histological and ultrastructural characterization of cytotoxic effects of amitraz on the ovaries of engorged females of Rhipicephalus (Boophilus) annulatus. Exp. Parasitol. 2019, 204, 107732. [Google Scholar] [CrossRef]
- Ajith, K.K.; Fular, A.; Chigure, G.; Sharma, A.K.; Nagar, G.; Souza, F.F.; Bechara, G.H.; Ghosh, S. Comparative impact of coumaphos, amitraz and plant extract of Ageratum conyzoides on the oogenesis of Rhipicephalus microplus. Ticks Tick Borne Dis. 2019, 10, 1085–1095. [Google Scholar] [CrossRef]
- Rani, N.P.; Moorthi, C.; Senthamarai, R.; Kathiresan, K. A study to explore the pharmacognostic and phytochemical screening of Artemisia nilagirica leaves found in Nilgiris district of Tamil Nadu. Int. J. Pharm. Pharm. Sci. 2012, 4, 441–447. [Google Scholar]
- Sati, S.C.; Sati, N.; Ahluwalia, V.; Walia, S.; Sati, O.P. Chemical composition and antifungal activity of Artemisia nilagirica essential oil growing in northern hilly areas of India. Nat. Prod. Res. 2012, 27, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, C.; Murugan, K.; Kovendan, K.; Kumar, P.M. Mosquito larvicidal, pupicidal, adulticidal, and repellent activity of Artemisia nilagirica (Family: Compositae) against Anopheles stephensi and Aedes aegypti. Parasitol. Res. 2012, 111, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Udayan, D.; Nair, S.N.; Juliet, S.; Ravindran, R.; Athalathil, S.; Adarshkrishna, T.P.; Ajith, K.G.; Sreelekha, K.P.; Ghosh, S. Acaricidal activity of Artemisia nilagirica leaves against Rhipicephalus (Boophilus) annulatus ticks. Planta Med. 2020, 86, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Ivănescu, B.; Burlec, A.F.; Crivoi, F.; Rosu, C.; Corciovă, A. Secondary Metabolites from Artemisia genus as biopesticides and innovative nano-based application strategies. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef]
- Qu, Z.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. Juvenile hormone and sesquiterpenoids in arthropods: Biosynthesis, signaling, and role of MicroRNA. J. Steroid. Biochem. Mol. Biol. 2018, 184, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.H. Hormonal control of tick development and reproduction. Parasitology 2004, 129, S127–S143. [Google Scholar] [CrossRef] [Green Version]
- Bowman, A.; Nuttall, P. Ticks: Biology, Disease and Control, 1st ed.; Cambridge University Press: Cambridge, UK, 2009; p. 506. [Google Scholar]
- Kaufman, W.B. Factors That Determine Sperm Precedence in Ticks, Spiders and Insects: A Comparative Study. In Ticks: Biology, Disease and Control, 1st ed.; Bowman, A., Nuttall, P., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 164–184. [Google Scholar]
- Spaziani, E.; Wang, W.L. Biosynthesis of ecdysteroid hormones by crustacean Y-organs: Conversion of cholesterol to 7-dehydrocholesterol is suppressed by a steroid 5α-reductase inhibitor. Mol. Cell Endocrinol. 1993, 95, 111–114. [Google Scholar] [CrossRef]
- Tahara, D.; Yano, I. Maturation-related variations in prostaglandin and fatty acid content of ovary in the Kuruma prawn (Marsupenaeus japonicus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 631–637. [Google Scholar] [CrossRef]
- Stanley, D. Prostaglandins and other eicosanoids in insects: Biological significance. Annu. Rev. Entomol. 2006, 51, 25–44. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Prostaglandins and their receptors in insect biology. Exp. Endocrinol. 2011, 2, 105. [Google Scholar] [CrossRef] [Green Version]
- Sumpownon, C.; Engsusophon, A.; Siangchan, T.; Sugiyama, E.; Soonklang, N.; Meeratana, P.; Wanichanon, C.; Hanna, P.J.; Setou, M. Variation of prostaglandin E2 concentrations in ovaries and its effects on ovarian maturation and oocyte proliferation in the giant freshwater prawn, Macrobrachium rosenbergii. Gen. Comp. Endocrinol. 2015, 223, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Swetha, C.H.; Girish, B.P.; Hemalatha, M.; Sreenivasula, P.R. Induction of vitellogenesis, methyl farnesoate synthesis and ecdysteroidogenesis in two edible crabs by arachidonic acid and prostaglandins. J. Exp. Biol. 2020, 223, jeb212381. [Google Scholar] [CrossRef]
- Varvas, K.; Kurg, R.; Hansen, K.; Jarving, R.; Jarving, I.; Valmsen, K.; Lohelaid, H.; Samel, N. Direct evidence of the cyclooxygenase pathway of prostaglandin synthesis in arthropods: Genetic and biochemical characterization of two crustacean cyclooxygenases. Insect Biochem. Mol. Biol. 2009, 39, 851–860. [Google Scholar] [CrossRef]
- Porter, R.B.R.; Reese, P.B.; Lawrence, A.D.W.; David, J.W. Acaricidal and insecticidal activities of Cadina-4, 10 (15)-dien-3-one. Phytochemistry 1995, 40, 735–738. [Google Scholar] [CrossRef]
- Perri, F.; Frattaruolo, L.; Haworth, I.; Brindisi, M.; El-magboub, A.; Ferrario, A.; Gomer, C.; Aiello, F.; Adams, J.D. Naturally occurring sesquiterpene lactones and their semi-synthetic derivatives modulate PGE2 levels by decreasing COX2 activity and expression. Heliyon 2019, 5, e01366. [Google Scholar] [CrossRef] [Green Version]
- Suresh, J.; Mahesh, N.M.; Ahuja, J.; Santilna, K.S. Review on Artemisia nilagirica (Clarke) Pamp. JBAPN 2011, 1, 97–104. [Google Scholar] [CrossRef]
- María, J.A.; Luis, M.B.; Luis, A.; Paulina, B. The Artemisia, L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Shakila, R. Chromatographic studies on Artemisia nilagirica leaf volatile oil. AJPLS 2013, 3, 185–190. [Google Scholar]
- Gairola, S.; Shariff, N.M.; Bhatt, A. Influence of climate change on production of secondary chemicals in high altitude medicinal plants: Issues need immediate attention. J. Med. Plant Res. 2010, 4, 1825–1829. [Google Scholar] [CrossRef]
- Sahoo, K.P.; Kasera, P.K.; Mohammed, S. Secondary metabolites produced during different seasons in some arid medicinal plants. Asian J. Plant Sci. Res. 2012, 2, 650–652. [Google Scholar]
- Kriker, S.; Yahia, A.; Nebbache, S. Effect of climate on some morphological and chemical characteristics of the plant Glycyrrhiza glabra L. in two arid regions of southern Algeria. Egypt. Acad. J. Biol. Sci. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Haider, F.; Naqvi, A.A.; Bagchi, G.D. Oil constituents of Artemisia nilagirica var. septentrionalis during different growth phases at subtropical conditions of North Indian plains. J. Essent. Oil Res. 2007, 19, 5–7. [Google Scholar] [CrossRef]
- Mishra, T.; Srivastava, M.; Kumar, A.; Pal, M.; Tewari, S.K. Chemical composition and Termiticidal activity of Artemisia nilagirica Essential oil growing in Southern Hilly Regions of India. J. Essent. Oil Bear. Plants. 2017, 20, 247–252. [Google Scholar] [CrossRef]
- Pereira, J.R.; Famadas, K.M. The efficiency of extracts of Dahlstedtia pentaphylla (Leguminosae, Papilionoidae, Millettiedae) on Boophilus microplus (Canestrini, 1887) in artificially infested bovines. Vet. Parasitol. 2006, 142, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.D.F.; Bessa, P.A.D.; Freitas, E.D.P. Evaluation of activity of the crude ethanolic extract of Magonia pubescens St. Hil (Sapindaceae) against larvae of the cattle tick Rhipicephalus (Boophilus) microplus (Canestrini, 1887) (Acari: Ixodidae). Braz. Arch. Biol. Technol. 2007, 51, 1147–1152. [Google Scholar] [CrossRef]
- Ribeiro, V.L.S.; Toigo, E.; Bordignon, S.A.; Gonçalves, K.; Von Poser, G. Acaricidal properties of extracts from the aerial parts of Hypericum polyanthemum on the cattle tick Boophilus microplus. Vet. Parasitol. 2007, 147, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Magadum, S.; Mondal, D.B.; Ghosh, S. Comparative efficacy of Annona squamosa and Azadirachta indica extracts against Boophilus microplus Izatnagar isolate. Parasitol. Res. 2009, 105, 1085–1091. [Google Scholar] [CrossRef]
- Drisya, K. GCMS Analysis of Bioactive Terpenoids Isolated From Essential Oil and Hexane Extract of Artemisia nilagirica Leaves. Master’s Dissertation, University of Calicut, Calicut, India, 2015; p. 24. [Google Scholar]
- Kanapadinchareveetil, S.; Chandrasekhar, L.; Gopi, J.; Ranjan Lenka, D.; Vasu, A.; Gopalan, A.K.K.; Nair, S.N.; Ravindran, R.; Juliet, S.; Ghosh, S. Histoarchitecture of the ovary of Rhipicephalus (Boophilus) annulatus during pre-and postengorgement period. Sci. World J. 2015, 2015, 126584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesen, K.J.; Kaufman, W.R. Cypermethrin inhibits egg development in the ixodid tick, Amblyomma hebraeum. Pestic. Biochem. Physiol. 2003, 76, 25–35. [Google Scholar] [CrossRef]
- Mordue, A.J.; Nisbet, A. Azadirachtin from the Neem tree Azadirachta indica: Its action against insects. An. Soc. Entomol. Brasil 2000, 29, 615–632. [Google Scholar] [CrossRef] [Green Version]
- Sales, K.J.; Jabbour, H.N. Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction 2003, 126, 559–567. [Google Scholar] [CrossRef]
- Kudo, I.; Murakami, M. Prostaglandin E synthase, a terminal enzyme for prostaglandin E2 biosynthesis. BMB Rep. 2005, 38, 633–638. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Kudo, I. Prostaglandin E synthase: A novel drug target for inflammation and cancer. Curr. Pharm. Des. 2006, 12, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Rodler, D.; Sinowatz, F. Expression of prostaglandin synthesizing enzymes (cyclooxygenase 1 and cyclooxygenase 2) in the ovary of the ostrich (Struthio camelus). Acta Histochemical. 2015, 117, 69–75. [Google Scholar] [CrossRef]
- Murakami, M.; Yoshihito, N.; Toshihiro, T.; Ichiro, K. Prostaglandin E synthase. Prostaglandins Lipid Mediat. 2002, 68, 383–399. [Google Scholar] [CrossRef]
- Enayatmehr, M.; Jamili, S. Prostaglandins and its role in aquatic reproduction: A Review. IJSR 2013, 4, 1238–1246. [Google Scholar]
- Destephano, D.B.; Brady, U.E. Prostaglandin and prostaglandin synthetase in the cricket Acheta domesticus. J. Insect Physiol. 1977, 23, 905–911. [Google Scholar] [CrossRef]
- Inokuma, H.; Kemp, D.H.; Willadsen, P. Comparison of prostaglandin E2 (PGE2) in salivary gland of Boophilus microplus, Haemaphysalis longicornis and Ixodes holocyclus, and quantification of PGE2 in saliva, hemolymph, ovary and gut of B. microplus. J. Vet. Med. Sci. 1994, 56, 1217–1218. [Google Scholar] [CrossRef] [Green Version]
- Sreelekha, K.P. Identification, Isolation and Characterization of Potential Acaricidal Molecule(s) from Plant Extract and Studies on Their Mode of Action. Ph.D. Thesis, Kerala Veterinary and Animal Sciences University, Pookode, India, 2015; p. 146. [Google Scholar]
- Spaziani, E.P.; Hinsch, G.W. Variation in selected unsaturated fatty acids during vitellogenesis in the Florida freshwater crayfish Procambarus paeninsulanus. Invertebr. Reprod. Dev. 1997, 32, 21–25. [Google Scholar] [CrossRef]
- Wimuttisuk, W.; Tobwor, P.; Deenarn, P.; Danwisetkanjana, K.; Pinkaew, D.; Kirtikara, K.; Vichai, V. Insights into the prostanoid pathway in the ovary development of the penaeid shrimp Penaeus monodon. PLoS ONE 2013, 8, e76934. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.D., III; Sonenshine, D.E.; Pérez de León, A.A. Vitellogenin receptor as a target for tick control: A mini-review. Front. Physiol. 2019, 10, 618. [Google Scholar] [CrossRef]
- Friesen, K.J.; Kaufman, W.R. Quantification of vitellogenesis and its control by 20-hydroxyecdysone in the ixodid tick, Amblyomma hebraeum. J. Insect Physiol. 2002, 48, 773–782. [Google Scholar] [CrossRef]
- Xavier, M.A.; Tirloni, L.; Pinto, A.F.; Diedrich, J.K.; Yates, J.R.; Mulenga, A.; Logullo, C.; da Silva Vaz, I.; Seixas, A.; Termignoni, C. A proteomic insight into vitellogenesis during tick ovary maturation. Sci. Rep. 2018, 8, 4698. [Google Scholar] [CrossRef]
- Iranshahi, M.; Emami, S.A.; Mahmoud-Soltani, M. Detection of sesquiterpene lactones in ten Artemisia species population of Khorasan provinces. Iran. J. Basic. Med. Sci. 2007, 10, 183–188. [Google Scholar]
- Drummond, R.; Ernst, S.E.; Trevino, J.L.; Gladney, W.J.; Graham, O.H. Boophilus annulatus and Boophilus microplus: Laboratory tests for insecticides. J. Econ. Entomol. 1973, 66, 130–133. [Google Scholar] [CrossRef]
- FAO. Resistance Management and Integrated Parasite Control in Ruminants: Guidelines. Animal Production and Health Division; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; pp. 25–77. [Google Scholar]
- Finney, D.J. Probit Analysis-A Statistical Treatment of the Sigmoid Response Curve, 2nd ed.; Cambridge University Press: Cambridge, MA, USA, 1952; p. 318. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1994; pp. 217–252. [Google Scholar]



| Peak No. | Name of Compound | RT | Area % |
|---|---|---|---|
| 1 | Neoisothujyl alcohol or neoisothujol | 4.541 | 4.19 |
| 2 | Ledol | 10.139 | 1.11 |
| 3 | Cycloisolongifolene, 8,9-dehydro- | 10.34 | 2.05 |
| 4 | beta-eudesmol | 10.71 | 2.72 |
| 5 | Longipinocarveol, trans- | 11.624 | 1.7 |
| 6 | Z,Z-2,15-Octadecedien-1-ol acetate | 12.7 | 2.29 |
| 7 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester (diisobutyl phthalate) | 12.945 | 2.03 |
| 8 | 3-Thujanone | 11.309 | 0.54 |
| 9 | Isothujol | 12.135 | 1.83 |
| 10 | Isothujol | 13.01 | 7.46 |
| 11 | trans-3(10)-Caren-2-ol | 16.215 | 0.32 |
| 12 | Spathulenol(1H-Cycloprop[e]azulen-7-ol) | 19.202 | 0.99 |
| 13 | Caryophyllene oxide | 19.845 | 0.84 |
| 14 | Ledol | 21.047 | 0.44 |
| 15 | Tetracyclo [6.3.2.0(2,5).0(1,8)]tridecan-9-ol,4,4-di methyl- | 21.724 | 1.99 |
| 16 | 4-epi-cubedol | 21.79 | 0.7 |
| 17 | β-Eudesmol | 21.885 | 7.05 |
| 18 | Caryophyllene oxide | 21.955 | 0.96 |
| 19 | tau.-Cadinol | 22.125 | 0.45 |
| 20 | 4,6,6-Trimethyl-2-(3-methylbuta-1,3-dienyl)-3-oxatricyclo[5.1.0.0(2,4)]octane | 22.292 | 0.54 |
| 21 | 9,10-Dimethyltricyclo[4.2.1.1(2,5)]decane-9,10-diol | 22.545 | 1.03 |
| 22 | (−)-Spathulenol | 23.11 | 1.78 |
| 23 | Nootkaton-11,12-epoxide | 23.415 | 1.09 |
| 24 | Longifolene-(I2)-epoxide-(1) | 24.129 | 1.55 |
| 25 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester (diisobutyl phthalate) | 24.172 | 4.87 |
| 26 | alpha-Copaen-11-ol(Tricyclo[4.4.0.0(2,7)]dec-8-ene-3-methanol) | 24.293 | 0.74 |
| 27 | Phytol (3,7,11,15-Tetramethyl-2-hexadecen-1-ol) | 24.409 | 1.37 |
| 28 | Platambin | 24.546 | 1.38 |
| 29 | Alloaromadendrene oxide-(1) | 24.604 | 0.4 |
| 30 | Ethyl palmitate (Hexadecanoic acid, ethyl ester) | 25.438 | 4.79 |
| 31 | Phytol | 26.854 | 4.36 |
| Sl. | Acaricide | Mean Ticks Weight per Replicate ± SEM | Mean (%) Adult Mortality within 15 Days ± SEM | Mean Eggs Mass per Replicate ± SEM | Index of Fecundity ± SEM | Percentage Inhibition of Fecundity (%) | Hatching (%) |
|---|---|---|---|---|---|---|---|
| No. | (g) | (g) | (Visual) | ||||
| 1 | Methanol (control) | 0.94 ± 0.02 a | 0 ± 0 a | 0.32 ± 0.06 a | 0.35 ± 0.06 a | 0 | 100% |
| 2 | 10% | 0.98 ± 0.00 a | 25 ± 8.33 c | 0.32 ± 0.03 a | 0.33 ± 0.03 a | 5.5 | 50% |
| 3 | 20% | 0.95 ± 0.00 a | 16.66 ± 6.80 d | 0.3 ± 0.05 a | 0.31 ± 0.06 a | 4.1 | 50% |
| 4 | 30% | 0.97 ± 0.01 a | 16.67 ± 11.79 d | 0.28 ± 0.02 a | 0.29 ± 0.02 b | 11.82 | 25% |
| 5 | 40% | 0.94 ± 0.02 a | 66.66 ± 6.80 b | 0.11 ± 0.01 b | 0.12 ± 0.01 c | 62.85 | 5% |
| 6 | 50% | 0.93 ± 0.02 a | 95.83 ± 4.17 a | 0.02 ± 0.01 b | 0.02 ± 0.01 d | 94.19 | 0 |
| Treated Groups | PGE2 Concentration (pg/tick) (n = 6; Mean ± SE) |
|---|---|
| Methanol (control) | 24.1603 ± 2.484 a |
| Flumethrin | BDL |
| Terpenoid subfraction | 4.5448 ± 0.074 b |
| Treated Group | Optical Density Values (n = 6; Mean ± SEM) |
|---|---|
| Methanol (Control) | 0.1417 ± 0.0050 a |
| Flumethrin | 0.1503 ± 0.0087 a |
| Terpenoid subfraction | 0.1525 ± 0.0037 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramachandran, P.D.; Muniyappa, M.D.; Kanapadinchareveetil, S.; Nair, S.N.; Ajithkumar, K.G.; Samraj, S.; Rajappan, A.; Varghese, A.; Kalarickal, D.C.; Ravindran, R.; et al. Modulation of the PGE2-Mediated Pathway in the Eclosion Blocking Effect of Flumethrin and Terpenoid Subfraction Isolated from Artemesia nilagirica in Rhipicephalus annulatus. Molecules 2021, 26, 4905. https://doi.org/10.3390/molecules26164905
Ramachandran PD, Muniyappa MD, Kanapadinchareveetil S, Nair SN, Ajithkumar KG, Samraj S, Rajappan A, Varghese A, Kalarickal DC, Ravindran R, et al. Modulation of the PGE2-Mediated Pathway in the Eclosion Blocking Effect of Flumethrin and Terpenoid Subfraction Isolated from Artemesia nilagirica in Rhipicephalus annulatus. Molecules. 2021; 26(16):4905. https://doi.org/10.3390/molecules26164905
Chicago/Turabian StyleRamachandran, Panicker Devyani, Mahesh Doddadasarahalli Muniyappa, Sreelekha Kanapadinchareveetil, Suresh Narayanan Nair, Karapparambu Gopalan Ajithkumar, Sujith Samraj, Anoopraj Rajappan, Anju Varghese, Deepa Chundayil Kalarickal, Reghu Ravindran, and et al. 2021. "Modulation of the PGE2-Mediated Pathway in the Eclosion Blocking Effect of Flumethrin and Terpenoid Subfraction Isolated from Artemesia nilagirica in Rhipicephalus annulatus" Molecules 26, no. 16: 4905. https://doi.org/10.3390/molecules26164905
APA StyleRamachandran, P. D., Muniyappa, M. D., Kanapadinchareveetil, S., Nair, S. N., Ajithkumar, K. G., Samraj, S., Rajappan, A., Varghese, A., Kalarickal, D. C., Ravindran, R., Ghosh, S., & Juliet, S. (2021). Modulation of the PGE2-Mediated Pathway in the Eclosion Blocking Effect of Flumethrin and Terpenoid Subfraction Isolated from Artemesia nilagirica in Rhipicephalus annulatus. Molecules, 26(16), 4905. https://doi.org/10.3390/molecules26164905