Sheep Wool Humidity under Electron Irradiation Affects Wool Sorptivity towards Co(II) Ions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Time Dependence of Sorptivity and Development of S-Oxidized Products at Different Absorbed Doses
2.1.1. Non-Irradiated Wool (0 kGy)
2.1.2. Wool with an Absorbed Dose of 109 kGy
2.1.3. Wool with an Absorbed Dose of 257 kGy
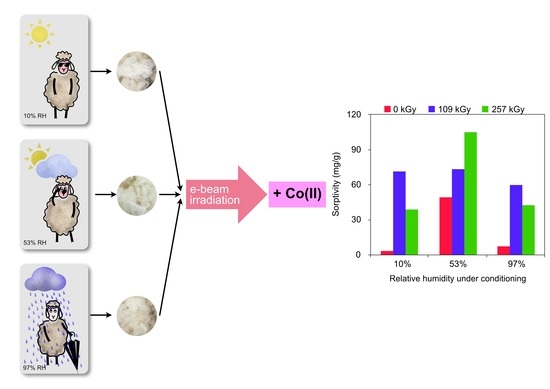
2.2. Dependence of Sorptivity on Dose for Various Conditioning Relative Humidity
2.2.1. Wool Conditioned at 10% RH
2.2.2. Wool Conditioned at 53% RH
2.2.3. Wool Conditioned at 97% RH
| (a) | Splitting of –S–S– bond → –S* + –S* | ~429 kJ/mol [40] |
| (b) | The splitting of hydroxyl OH− → H+ + O2− | ~429 kJ/mol [40] |
| (c) | The splitting (radiolysis) of H2O → OH− + H+ | ~499 kJ/mol [40] |
| (d) | The progressive oxidation –S products to cysteic acid [25] | |
| … cystine dioxide | ||
| The above S-product are step by step transformed to cysteic acid R-SO3. | ||
2.3. Dependence of pH in Wool Extract on Absorbed Dose at Various Conditioning RH
2.3.1. Values of pH in Extracts from Non-Irradiated Wool
2.3.2. Values of pH in Extracts from Wool Dosed 109 kGy
2.3.3. Values of pH in Extracts from Wool Dosed 257 kGy
2.4. Summary of Sorption Results Following Conditioning Conditions
3. Material and Methods
3.1. Materials
3.2. Sample Conditioning and Irradiation
3.3. Sorption Experiments
3.4. Visible Spectral Analysis
3.5. FTIR Spectral Analysis
3.6. Measurement of pH
4. Conclusions
- ○
- Current humidity of wool under the irradiation.
- ○
- Content of both cysteic acid and cystine monoxide.
- ○
- Absorbed dose; the highest applied dose of 257 kGy causes radiolysis of superfluous water and reduces Co(II) sorption.
- ○
- The post-exposure time; within a monitored lapse of 160 days after exposure, the Co(II) sorptivity increased with time only for wool conditioned at 53% RH, at all absorbed doses. This finding is an important merit for practice, eliminating the need for pre-exposure wool conditioning.
- ○
- The wool conditioned at 53% RH and dosed 257 kGy has shown the highest sorptivity; this humidity is close to that of the common environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jóźwiak-Niedźwiedzka, D.; Alessandro, P.; Fantill, A.P. Wool-reinforced cement based composites. Materials 2020, 13, 3590. [Google Scholar] [CrossRef]
- Galán-Marín, C.; Rivera-Gómez, C.; Petric-Gray, J. Effect of animal fibres reinforcement on stabilized earth mechanical properties. J. Biobased Mater. Bioenergy 2010, 4, 121–128. [Google Scholar] [CrossRef]
- Ghosh, A.; Collie, S. Keratinous materials as novel absorbent systems for toxic pollutants. Def. Sci. J. 2014, 64, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Wen, G.; Naik, R.; Cookson, P.; Smith, S.; Liu, X.; Wang, X. Wool powders used as sorbents to remove Co2+ ions from aqueous solution. Powder Technol. 2010, 197, 235–240. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Salama, M.; Kantouch, A.A.M. Wool micro powder as a metal ion exchanger for the removal of copper and zinc. Desalin. Water. Treat. 2015, 56, 1010–1019. [Google Scholar] [CrossRef]
- Balkaya, N.; Bektas, N. Chromium (VI) sorption from dilute aqueous solutions using wool. Desalin. Water. Treat. 2009, 3, 43–49. [Google Scholar] [CrossRef]
- Balköse, D.; Baltacioǧlu, H.; Baltacioğlu, H. Adsorption of heavy metal cations from aqueous solutions by wool fibers. J. Chem. Technol. Biotechnol. 2007, 54, 393–397. [Google Scholar] [CrossRef]
- Mažeikienė, A.; Vaiškūnaitė, R.; Vaišis, V. Oil removal from runoff with natural sorbing filter fillers. J. Environ. Manag. 2014, 141, 155–160. [Google Scholar] [CrossRef]
- Rajakovic, V.; Aleksic, G.; Radetic, M.; Rajakovic, L. Efficiency of oil removal from real wastewater with different sorbent materials. J. Hazard. Mater. 2007, 143, 494–499. [Google Scholar] [CrossRef]
- Cieślak, M.; Schmidt, H. Contamination of wool fibre exposed to environmental tobacco smoke. Fibres Text. East. Eur. 2004, 12, 81–83. [Google Scholar]
- Curling, S.F.; Loxton, C.; Ormondroyd, G.A. A rapid method for investigation the absorption of formaldehyde from air by wool. J. Mater. Sci. 2012, 47, 3248–3251. [Google Scholar] [CrossRef]
- Freddi, G.; Arai, T.; Colonna, G.M.; Boschi, A.; Tsukada, M. Binding of metal cations to chemically modified wool and antimicrobial properties of the wool–metal complexes. J. Appl. Polym. Sci. 2001, 82, 3513–3519. [Google Scholar] [CrossRef]
- Taddei, P.; Monti, P.; Freddi, G.; Arai, T.; Tsukada, M. Binding of Co (II) and Cu (II) cations to chemically modified wool fibres. An IR investigation. J. Mol. Struct. 2003, 650, 105–113. [Google Scholar] [CrossRef]
- Nikiforova, T.E.; Kozlov, V.A.; Sionikhina, A.N. Peculiarities of sorption of copper (II) ions by modified wool keratin. Prot. Met. Phys. Chem. 2019, 55, 849–857. [Google Scholar] [CrossRef]
- Monier, M.; Ayad, M.D.; Sarhan, A.A. Adsorption of Cu (II), Hg (II), and Ni (II) ions by modified natural wool chelating fibers. J. Hazard. Mater. 2010, 176, 348–355. [Google Scholar] [CrossRef]
- Evangelou, M.W.; Ebel, M.; Koerner, A.; Schaeffer, A. Hydrolysed wool: A novel chelating agent for metal chelant-assisted phytoextraction from soil. Chemosphere 2008, 72, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, A.H.H.; Salem, T.; Radwan, E.K.; El-Sayed, A.A.; Embaby, M. Modification of waste wool fiber as low cost adsorbent for the removal of methylene blue from aqueous solution. Egypt. J. Chem. 2017, 60, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Kan, C.W.; Chan, K.; Yuen, C.W.M.; Miao, M.H. Surface properties of low-temperature plasma treated wool fabrics. J. Mater. Process. Technol. 1998, 83, 180–184. [Google Scholar] [CrossRef]
- Kan, C.-W.; Yuen, C.W.M. Surface characterisation of low temperature plasma-treated wool fibre. J. Mater. Process. Technol. 2006, 178, 52–60. [Google Scholar] [CrossRef]
- Kan, C.-W.; Yuen, C.-W.M. Plasma technology in wool. Tex. Prog. 2007, 39, 121–187. [Google Scholar] [CrossRef]
- Ceria, A.; Rombaldoni, F.; Rovero, G.; Mazzuchetti, G.; Sicardi, S. The effect of an innovative atmospheric plasma jet treatment on physical and mechanical properties of wool fabrics. J. Mater. Process. Technol. 2010, 210, 720–726. [Google Scholar] [CrossRef]
- Xu, W.; Shen, X.; Wang, X.; Ke, G. Effective methods for further improving the wool properties treated by corona discharge. Sen’i Gakkaishi 2006, 62, 111–114. [Google Scholar] [CrossRef]
- Ke, G.; Yu, W.; Xu, W.; Cui, W.; Shen, X. Effect of corona discharge treatment on the surface properties of wool fabrics. J. Mater. Process. Technol. 2008, 207, 125–129. [Google Scholar] [CrossRef]
- Fakin, D.; Ojstršek, A.; Čelan Benkovič, S. The impact of corona modifies fibres’ chemical changes on wool dyening. J. Mater. Process. Technol. 2009, 209, 584–589. [Google Scholar] [CrossRef]
- Porubská, M.; Hanzlíková, Z.; Braniša, J.; Kleinová, A.; Hybler, P.; Fülöp, M.; Ondruška, J.; Jomová, K. The effect of electron beam on sheep wool. Polym. Degrad. Stabil. 2015, 111, 151–158. [Google Scholar] [CrossRef]
- Directive 1999/2/EC of the European Parliament and of the Council of 22 February 1999 on the Approximation of the Laws of the Member States Concerning Foods and Food Ingredients Treated with Ionising Radiation. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A31999L0002 (accessed on 10 February 2021).
- McNeal, T.P.; Komolprasert, V.; Buchalla, R.; Olivo, C.; Begley, T.H. Effects of ionizing radiation on food contact materials. ACS Symp. Ser. 2004, 875, 214–235. [Google Scholar]
- Dadbin, S.; Frounchi, M.; Goudarzi, D. Electron beam induced crosslinking of nylon 6 with and without the presence of TAC. Polym. Degrad. Stabil. 2005, 89, 436–441. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Lawson, M.K.; Hybler, P.; Fülöp, M.; Porubská, M. Time-dependent variations in structure of sheep wool irradiated by electron beam. Adv. Mater. Sci. Eng. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hanzlíková, Z.; Braniša, J.; Hybler, P.; Šprinclová, I.; Jomová, K.; Porubská, M. Sorption properties of sheep wool irradiated by accelerated electron beam. Chem. Pap. 2016, 70, 299–1308. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Braniša, J.; Jomová, K.; Fülöp, M.; Hybler, P.; Porubská, M. Electron beam irradiated sheep wool—Prospective sorbent for heavy metals in wastewater. Separ. Purif. Technol. 2018, 193, 345–350. [Google Scholar] [CrossRef]
- Braniša, J.; Jomová, K.; Kovalčíková, R.; Hybler, P.; Porubská, M. Role of post-exposure time in Co (II) sorption of higher concentrations on electron irradiated sheep wool. Molecules 2019, 24, 2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanzlíková, Z.; Braniša, J.; Ondruška, J.; Porubská, M. The uptake and release of humidity by wool irradiated with electron beam. J. Central Eur. Agric. 2016, 17, 315–324. [Google Scholar] [CrossRef]
- Porubská, M.; Kleinová, A.; Hybler, P.; Braniša, J. Why natural or electron irradiated sheep wool show anomalous sorption of higher concentrations of copper (II). Molecules 2018, 23, 3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oae, S. Organic Sulfur Chemistry: Structure and Mechanism, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991; p. 433. ISBN 0-8493-4739-4. [Google Scholar]
- Porubská, M.; Janigová, I.; Jomová, K.; Chodák, I. The effect of electron beam irradiation on properties of virgin and glass fiber-reinforced polyamide 6. Radiat. Phys. Chem. 2014, 102, 159–166. [Google Scholar] [CrossRef]
- Sengupta, R.; Tikku, V.; Somani, A.K.; Chaki, T.K.; Bhowmick, A.K. Electron beam irradiated polyamide-6,6 films—I: Characterization by wide angle X-ray scattering and infrared spectroscopy. Radiat. Phys. Chem. 2005, 72, 625–633. [Google Scholar] [CrossRef]
- Pramanik, N.K.; Haldar, R.; Bhardwaj, Y.; Sabharwal, S.; Niyogi, U.; Khandal, R. Radiation processing of Nylon 6 by e-beam for improved properties and performance. Radiat. Phys. Chem. 2009, 78, 199–205. [Google Scholar] [CrossRef]
- Project, No. 01TU Z-4/2016: Interactive Monitor of Dryness—Tool for Transfer of Knowledge about Risk in Country for Teaching and Real Practice (in Slovak). Available online: https://bioclio.com/vlhkost-vzduchu-2/ (accessed on 10 February 2021).
- de Darwent, B. (Ed.) Bond Dissociation Energies in Simple Molecules; NSRDS-NBS 31; National Bureau of Standards (U.S.), Department of Chemistry: Washington, DC, USA, 1970; pp. 41–44. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/NSRDS/nbsnsrds31.pdf (accessed on 10 February 2021).
- Braniša, J.; Kleinová, A.; Jomová, K.; Malá, R.; Morgunov, V.; Porubská, M. Some properties of electron beam-irradiated sheep wool linked to Cr (III) sorption. Molecules 2019, 24, 4401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81, 89. [Google Scholar] [CrossRef]











| Bond | Reaction | Dissociation Energy at 298 K (kJ/mol) |
|---|---|---|
| H2O | H2O → OH + H | 498.7 ± 0.08 |
| H-O | OH → H + O | 428.0 ± 2.1 |
| S-S | S-S → S + S | 428.9 ± 6.3 |
| Saturated Aqueous Solution | Relative Humidity (%) |
|---|---|
| KOH | 9.32 ± 0.9 |
| Mg(NO3)2 | 53.38 ± 0.23 |
| K2SO4 | 97.59 ± 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braniša, J.; Kleinová, A.; Jomová, K.; Weissabel, R.; Cvik, M.; Branišová, Z.; Porubská, M. Sheep Wool Humidity under Electron Irradiation Affects Wool Sorptivity towards Co(II) Ions. Molecules 2021, 26, 5206. https://doi.org/10.3390/molecules26175206
Braniša J, Kleinová A, Jomová K, Weissabel R, Cvik M, Branišová Z, Porubská M. Sheep Wool Humidity under Electron Irradiation Affects Wool Sorptivity towards Co(II) Ions. Molecules. 2021; 26(17):5206. https://doi.org/10.3390/molecules26175206
Chicago/Turabian StyleBraniša, Jana, Angela Kleinová, Klaudia Jomová, Róbert Weissabel, Marcel Cvik, Zuzana Branišová, and Mária Porubská. 2021. "Sheep Wool Humidity under Electron Irradiation Affects Wool Sorptivity towards Co(II) Ions" Molecules 26, no. 17: 5206. https://doi.org/10.3390/molecules26175206
APA StyleBraniša, J., Kleinová, A., Jomová, K., Weissabel, R., Cvik, M., Branišová, Z., & Porubská, M. (2021). Sheep Wool Humidity under Electron Irradiation Affects Wool Sorptivity towards Co(II) Ions. Molecules, 26(17), 5206. https://doi.org/10.3390/molecules26175206