Amorphous Form of Carvedilol Phosphate—The Case of Divergent Properties
Abstract
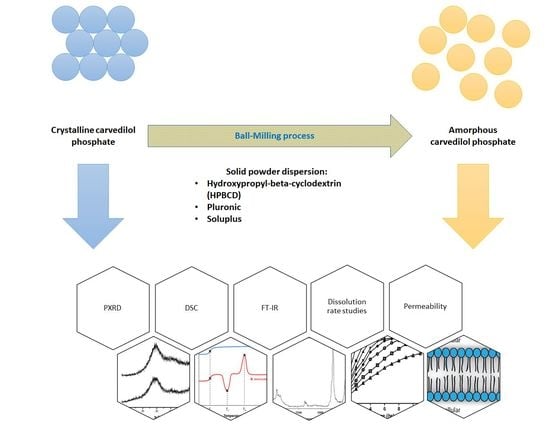
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Amorphous Form of Carvedilol Phosphate
3.3. Powder X-ray Diffraction (PXRD)
3.4. Different Scanning Calorimetry (DSC)
3.5. FT-IR Spectroscopy and Density Functional Theory (DFT)
3.6. Preparation of Physical Mixtures of CVD with Excipients
3.7. UHPLC-DAD Method
3.8. Apparent Solubility Studies
3.9. Permeability Studies of Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Singh, S.; Preuss, C.V. Carvedilol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ruffolo, R.R.; Feuerstein, G.Z. Carvedilol: Preclinical Profile and Mechanisms of Action in Preventing the Progression of Congestive Heart Failure. Eur. Heart J. 1998, 19 (Suppl. B), B19–B24. [Google Scholar]
- Rasool, M.F.; Läer, S. Development and Evaluation of a Physiologically Based Pharmacokinetic Model to Predict Carvedilol-Paroxetine Metabolic Drug-Drug Interaction in Healthy Adults and Its Extrapolation to Virtual Chronic Heart Failure Patients for Dose Optimization. Expert Opin. Drug Metab. Toxicol. 2021, 17, 717–724. [Google Scholar] [CrossRef]
- Cardoso, J.; de Espíndola, M.D.; Cunha, M.; Netto, E.; Cardoso, C.; Novaes, M.; Del Carlo, C.H.; Brancalhão, E.; Name, A.L.; Barretto, A.C.P. Is Current Drug Therapy for Heart Failure Sufficient to Control Heart Rate of Patients? Arq. Bras. Cardiol. 2020, 115, 1063–1069. [Google Scholar] [CrossRef]
- Vögele, A.; Johansson, T.; Renom-Guiteras, A.; Reeves, D.; Rieckert, A.; Schlender, L.; Teichmann, A.-L.; Sönnichsen, A.; Martinez, Y.V. Effectiveness and Safety of Beta Blockers in the Management of Hypertension in Older Adults: A Systematic Review to Help Reduce Inappropriate Prescribing. BMC Geriatr. 2017, 17, 224. [Google Scholar] [CrossRef]
- Fernandes, G.J.; Kumar, L.; Sharma, K.; Tunge, R.; Rathnanand, M. A Review on Solubility Enhancement of Carvedilol—A BCS Class II Drug. J. Pharm. Innov. 2018, 13, 197–212. [Google Scholar] [CrossRef]
- Elgart, A.; Cherniakov, I.; Aldouby, Y.; Domb, A.J.; Hoffman, A. Improved Oral Bioavailability of BCS Class 2 Compounds by Self Nano-Emulsifying Drug Delivery Systems (SNEDDS): The Underlying Mechanisms for Amiodarone and Talinolol. Pharm. Res. 2013, 30, 3029–3044. [Google Scholar] [CrossRef]
- Neelam, S.; Allawadi, D.; Singh, S.; Sandeep, A. Techniques for Bioavailability Enhancement of BCS Class II Drugs: A Review. Int. J. Pharm. Chem. Sci. 2013, 2, 1092–1101. [Google Scholar]
- von Möllendorff, E.; Reiff, K.; Neugebauer, G. Pharmacokinetics and Bioavailability of Carvedilol, a Vasodilating Beta-Blocker. Eur. J. Clin. Pharmacol. 1987, 33, 511–513. [Google Scholar] [CrossRef]
- Einfal, T.; Planinšek, O.; Hrovat, K. Methods of Amorphization and Investigation of the Amorphous State. Acta Pharm. 2013, 63, 305–334. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Grohganz, H.; Löbmann, K.; Rades, T.; Hempel, N.-J. Co-Amorphous Drug Formulations in Numbers: Recent Advances in Co-Amorphous Drug Formulations with Focus on Co-Formability, Molar Ratio, Preparation Methods, Physical Stability, In Vitro and In Vivo Performance, and New Formulation Strategies. Pharmaceutics 2021, 13, 389. [Google Scholar] [CrossRef]
- Thorat, Y.; Gonjari, I.; Hosmani, A. Solubility Enhancement Techniques: A Review On Conventional And Novel Approaches. Int. J. Pharm. Sci. Res. 2011, 2, 2501–2513. [Google Scholar]
- Mehta, S.; Joseph, N.M.; Feleke, F.; Palani, S. Improving Solubility of Bcs Class Ii Drugs Using Solid Dispersion: A Review. J. Drug Deliv. Ther. 2014, 4, 7–13. [Google Scholar] [CrossRef]
- Talaczynska, A.; Dzitko, J.; Cielecka-Piontek, J. Benefits and Limitations of Polymorphic and Amorphous Forms of Active Pharmaceutical Ingredients. Curr. Pharm. Des. 2016, 22, 4975–4980. [Google Scholar] [CrossRef]
- Rams-Baron, M.; Jachowicz, R.; Boldyreva, E.; Zhou, D.; Jamroz, W.; Paluch, M. Physical Instability: A Key Problem of Amorphous Drugs. In Amorphous Drugs: Benefits and Challenges; Rams-Baron, M., Jachowicz, R., Boldyreva, E., Zhou, D., Jamroz, W., Paluch, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 107–157. ISBN 978-3-319-72002-9. [Google Scholar]
- Skotnicki, M.; Apperley, D.C.; Aguilar, J.A.; Milanowski, B.; Pyda, M.; Hodgkinson, P. Characterization of Two Distinct Amorphous Forms of Valsartan by Solid-State NMR. Mol. Pharm. 2016, 13, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Law, D.; Schmitt, E.A.; Marsh, K.C.; Everitt, E.A.; Wang, W.; Fort, J.J.; Krill, S.L.; Qiu, Y. Ritonavir-PEG 8000 Amorphous Solid Dispersions: In Vitro and in Vivo Evaluations. J. Pharm. Sci. 2004, 93, 563–570. [Google Scholar] [CrossRef]
- Indulkar, A.S.; Lou, X.; Zhang, G.G.Z.; Taylor, L.S. Insights into the Dissolution Mechanism of Ritonavir–Copovidone Amorphous Solid Dispersions: Importance of Congruent Release for Enhanced Performance. Mol. Pharm. 2019, 16, 1327–1339. [Google Scholar] [CrossRef]
- Knapik-Kowalczuk, J.; Chmiel, K.; Pacułt, J.; Bialek, K.; Tajber, L.; Paluch, M. Enhancement of the Physical Stability of Amorphous Sildenafil in a Binary Mixture, with Either a Plasticizing or Antiplasticizing Compound. Pharmaceutics 2020, 12, 460. [Google Scholar] [CrossRef]
- Yani, Y.; Kanaujia, P.; Chow, P.S.; Tan, R.B.H. Effect of API-Polymer Miscibility and Interaction on the Stabilization of Amorphous Solid Dispersion: A Molecular Simulation Study. Ind. Eng. Chem. Res. 2017, 56, 12698–12707. [Google Scholar] [CrossRef]
- Lehmkemper, K.; Kyeremateng, S.O.; Bartels, M.; Degenhardt, M.; Sadowski, G. Physical Stability of API/Polymer-Blend Amorphous Solid Dispersions. Eur. J. Pharm. Biopharm. 2018, 124, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.B.; Rathi, S.; Jyothi, V.G.S.S.; Shastri, N.R. Cellulose Based Polymers in Development of Amorphous Solid Dispersions. Asian J. Pharm. Sci. 2019, 14, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Meng, K.; Chen, X.; Yao, H.; Kong, J.; Li, F.; Yin, H.; Jin, M.; Liang, H.; Yuan, Q. Preparation, Characterization, and In Vivo Evaluation of Amorphous Icaritin Nanoparticles Prepared by a Reactive Precipitation Technique. Molecules 2021, 26, 2913. [Google Scholar] [CrossRef]
- Pérez, Y.A.; Urista, C.M.; Martínez, J.I.; Nava, M.D.C.D.; Rodríguez, F.A.R. Functionalized Polymers for Enhance Oral Bioavailability of Sensitive Molecules. Polymers 2016, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.D.; Tran, P.H.L. Insoluble Polymers in Solid Dispersions for Improving Bioavailability of Poorly Water-Soluble Drugs. Polymers 2020, 12, 1679. [Google Scholar] [CrossRef]
- Krstic, M.; Djuris, J.; Petrovic, O.; Lazarevic, N.; Cvijic, S.; Ibric, S. Application of the Melt Granulation Technique in Development of Lipid Matrix Tablets with Immediate Release of Carbamazepine. J. Drug Deliv. Sci. Technol. 2017, 39, 467–474. [Google Scholar] [CrossRef]
- Krstić, M.; Ražić, S.; Djekic, L.; Dobričić, V.; Momčilović, M.; Vasiljevic, D.; Ibrić, S. Application of a Mixture Experimental Design in the Optimization of the Formulation of Solid Self-Emulsifying Drug Delivery Systems Containing Carbamazepine. Lat. Am. J. Pharm. 2015, 34, 885–894. [Google Scholar]
- Krstić, M.; Manić, L.; Martić, N.; Vasiljević, D.; Svetlana, Đ.; Vukmirović, S.; Raskovic, A. Binary Polymeric Amorphous Carvedilol Solid Dispersions: In Vitro and in Vivo Characterization. Eur. J. Pharm. Sci. 2020, 150, 105343. [Google Scholar] [CrossRef]
- Vogt, F.G.; Copley, R.C.B.; Mueller, R.L.; Spoors, G.P.; Cacchio, T.N.; Carlton, R.A.; Katrincic, L.M.; Kennady, J.M.; Parsons, S.; Chetina, O.V. Isomorphism, Disorder, and Hydration in the Crystal Structures of Racemic and Single-Enantiomer Carvedilol Phosphate. Cryst. Growth Des. 2010, 10, 2713–2733. [Google Scholar] [CrossRef]
- Bergese, P.; Colombo, I.; Gervasoni, D.; Depero, L.E. Assessment of the X-Ray Diffraction–Absorption Method for Quantitative Analysis of Largely Amorphous Pharmaceutical Composites. J. Appl. Cryst. 2003, 36, 74–79. [Google Scholar] [CrossRef]
- Descamps, M.; Dudognon, E. Crystallization from the Amorphous State: Nucleation–Growth Decoupling, Polymorphism Interplay, and the Role of Interfaces. J. Pharm. Sci. 2014, 103, 2615–2628. [Google Scholar] [CrossRef]
- Yoshioka, M.; Hancock, B.C.; Zografi, G. Crystallization of Indomethacin from the Amorphous State below and above Its Glass Transition Temperature. J. Pharm. Sci. 1994, 83, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Amorphous Pharmaceutical Solids: Preparation, Characterization and Stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Bannow, J.; Koren, L.; Salar-Behzadi, S.; Löbmann, K.; Zimmer, A.; Rades, T. Hot Melt Coating of Amorphous Carvedilol. Pharmaceutics 2020, 12, 519. [Google Scholar] [CrossRef]
- Pokharkar, V.B.; Mandpe, L.P.; Padamwar, M.N.; Ambike, A.A.; Mahadik, K.R.; Paradkar, A. Development, Characterization and Stabilization of Amorphous Form of a Low Tg Drug. Powder Technol. 2006, 167, 20–25. [Google Scholar] [CrossRef]
- Bhagat, C.; Singh, S.K.; Verma, P.R.P.; Singh, N.; Verma, S.; Ahsan, M.N. Crystalline and Amorphous Carvedilol-Loaded Nanoemulsions: Formulation Optimisation Using Response Surface Methodology. J. Exp. Nanosci. 2013, 8, 971–992. [Google Scholar] [CrossRef] [Green Version]
- Planinšek, O.; Kovačič, B.; Vrečer, F. Carvedilol Dissolution Improvement by Preparation of Solid Dispersions with Porous Silica. Int. J. Pharm. 2011, 406, 41–48. [Google Scholar] [CrossRef]
- Talik, P.; Hubicka, U. The DSC Approach to Study Non-Freezing Water Contents of Hydrated Hydroxypropylcellulose (HPC). J. Therm. Anal. Calorim. 2018, 132, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Shete, G.; Kuncham, S.; Puri, V.; Gangwal, R.P.; Sangamwar, A.T.; Bansal, A.K. Effect of Different “States” of Sorbed Water on Amorphous Celecoxib. J. Pharm. Sci. 2014, 103, 2033–2041. [Google Scholar] [CrossRef]
- Meng, F.; Ferreira, R.; Su, Y.; Zhang, F. A Novel Amorphous Solid Dispersion Based on Drug–Polymer Complexation. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Gui, Y.; McCann, E.C.; Yao, X.; Li, Y.; Jones, K.J.; Yu, L. Amorphous Drug–Polymer Salt with High Stability under Tropical Conditions and Fast Dissolution: The Case of Clofazimine and Poly(Acrylic Acid). Mol. Pharm. 2021, 18, 1364–1372. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yu, G.; Kim, I.W. Effects of Polymeric Additives on the Crystallization and Release Behavior of Amorphous Ibuprofen. J. Nanomater. 2013, 2013, e503069. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, C.; Chen, Z.; Su, C.; Hageman, M.; Hussain, M.; Haskell, R.; Stefanski, K.; Qian, F. Drug–Polymer–Water Interaction and Its Implication for the Dissolution Performance of Amorphous Solid Dispersions. Mol. Pharm. 2015, 12, 576–589. [Google Scholar] [CrossRef]
- Drygas, J.; Pawlowska, J.; Szramka, R.; Ramza, J. Amorphous Carvedilol Phosphate and Method of Manufacturing Thereof. U.S. Patent US20080242872A1, 10 February 2008. [Google Scholar]
- Sip, S.; Paczkowska-Walendowska, M.; Rosiak, N.; Miklaszewski, A.; Grabańska-Martyńska, K.; Samarzewska, K.; Cielecka-Piontek, J. Chitosan as Valuable Excipient for Oral and Topical Carvedilol Delivery Systems. Pharmaceuticals 2021, 14, 712. [Google Scholar] [CrossRef]
- Nolte, K.; Backfisch, G.; Neidlein, R. In Vitro Absorption Studies with Carvedilol Using a New Model with Porcine Intestine Called BM-RIMO (Boehringer-Mannheim Ring Model). Arzneimittelforschung 1999, 49, 745–749. [Google Scholar] [CrossRef]
- Carvedilol Tablets|USP-NF. Available online: https://www.uspnf.com/official-text/accelerated-revision-process/accelerated-revision-history/carvedilol-tablets (accessed on 1 August 2021).
- Morgan, T. Clinical Pharmacokinetics and Pharmacodynamics of Carvedilol. Clin. Pharm. 1994, 26, 335–346. [Google Scholar] [CrossRef]
- PubChem. Coreg CR. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11954344 (accessed on 1 August 2021).
- Chawla, G.; Bansal, A.K. Improved Dissolution of a Poorly Water Soluble Drug in Solid Dispersions with Polymeric and Non-Polymeric Hydrophilic Additives. Acta Pharm. 2008, 58, 257–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiergowska, G.; Ludowicz, D.; Wdowiak, K.; Miklaszewski, A.; Lewandowska, K.; Cielecka-Piontek, J. Combinations of Freeze-Dried Amorphous Vardenafil Hydrochloride with Saccharides as A Way to Enhance Dissolution Rate and Permeability. Pharmaceuticals 2021, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Knopp, M.M.; Olesen, N.E.; Holm, P.; Langguth, P.; Holm, R.; Rades, T. Influence of Polymer Molecular Weight on Drug–Polymer Solubility: A Comparison between Experimentally Determined Solubility in PVP and Prediction Derived from Solubility in Monomer. J. Pharm. Sci. 2015, 104, 2905–2912. [Google Scholar] [CrossRef] [Green Version]
- Karolewicz, B.; Gajda, M.; Górniak, A.; Owczarek, A.; Mucha, I. Pluronic F127 as a Suitable Carrier for Preparing the Imatinib Base Solid Dispersions and Its Potential in Development of a Modified Release Dosage Forms: Thermal, Spectroscopic, Microscopic, and Dissolution Studies. J. Therm. Anal. Calorim 2017, 130, 383–390. [Google Scholar] [CrossRef]
- Alopaeus, J.F.; Hagesæther, E.; Tho, I. Micellisation Mechanism and Behaviour of Soluplus®–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System. Pharmaceuticals 2019, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, B.L.A.; Sauceau, M.; Del Confetto, S.; Sescousse, R.; Ré, M.I. Determination of Drug-Polymer Solubility from Supersaturated Spray-Dried Amorphous Solid Dispersions: A Case Study with Efavirenz and Soluplus®. Eur. J. Pharm. Biopharm. 2019, 142, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of Increased Bioavailability through Amorphous Solid Dispersions: A Review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Markovic, M.; Zur, M.; Fine-Shamir, N.; Haimov, E.; González-Álvarez, I.; Dahan, A. Segmental-Dependent Solubility and Permeability as Key Factors Guiding Controlled Release Drug Product Development. Pharmaceutics 2020, 12, 295. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Nguyen, K.; Kerns, E.; Yan, Z.; Yu, K.R.; Shah, P.; Jadhav, A.; Xu, X. Highly Predictive and Interpretable Models for PAMPA Permeability. Bioorg. Med. Chem. 2017, 25, 1266–1276. [Google Scholar] [CrossRef] [Green Version]
- Yee, S. In Vitro Permeability across Caco-2 Cells (Colonic) Can Predict in Vivo (Small Intestinal) Absorption in Man--Fact or Myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef]
- Dahan, A.; Beig, A.; Lindley, D.; Miller, J.M. The Solubility-Permeability Interplay and Oral Drug Formulation Design: Two Heads Are Better than One. Adv. Drug Deliv. Rev. 2016, 101, 99–107. [Google Scholar] [CrossRef]
- Accounting for the solubility–permeability interplay in oral formulation development for poor water solubility drugs: The effect of PEG-400 on carbamazepine absorption. Eur. J. Pharm. Biopharm. 2012, 81, 386–391. [CrossRef]
- Volkova, T.V.; Domanina, E.N.; Kumeev, R.S.; Proshin, A.N.; Terekhova, I.V. The Effect of Different Polymers on the Solubility, Permeability and Distribution of Poor Soluble 1,2,4-Thiadiazole Derivative. J. Mol. Liq. 2018, 269, 492–500. [Google Scholar] [CrossRef]
- Mesallati, H.; Tajber, L. Polymer/Amorphous Salt Solid Dispersions of Ciprofloxacin. Pharm. Res. 2017, 34, 2425–2439. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Takata, N.; Yonemochi, E. Dose-Dependent Solubility–Permeability Interplay for Poorly Soluble Drugs under Non-Sink Conditions. Pharmaceutics 2021, 13, 323. [Google Scholar] [CrossRef]
- Nidhi, N.; Ranjit, S.; Sunil, J.; Vikas, A.S. The Solubility-Permeability Interplay for Solubility-Enabling Oral Formulations. Curr. Drug Targets 2019, 20, 1434–1446. [Google Scholar]
- McCallum, M.M. High-Throughput Approaches for the Assessment of Factors Influencing Bioavailability of Small Molecules in Pre-Clinical Drug Development. Ph.D. Thesis, The University of Wisconsin-Milwaukee, Milwaukee, WI, USA, 2013. [Google Scholar]
- Zhang, M.; Naik, R.R.; Dai, L. Carbon Nanomaterials for Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-22861-7. [Google Scholar]
- Ilie, A.-R.; Griffin, B.T.; Brandl, M.; Bauer-Brandl, A.; Jacobsen, A.-C.; Vertzoni, M.; Kuentz, M.; Kolakovic, R.; Holm, R. Exploring Impact of Supersaturated Lipid-Based Drug Delivery Systems of Celecoxib on in Vitro Permeation across Permeapad® Membrane and in Vivo Absorption. Eur. J. Pharm. Sci. 2020, 152, 105452. [Google Scholar] [CrossRef]
- Li, J.; Bukhtiyarov, Y.; Spivey, N.; Force, C.; Hidalgo, C.; Huang, Y.; Owen, A.J.; Hidalgo, I.J. In Vitro and In Vivo Assessment of the Potential of Supersaturation to Enhance the Absorption of Poorly Soluble Basic Drugs. J. Pharm. Innov. 2020, 15, 591–602. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Froehlich, C.; Kalia, Y.; Guy, R. Supersaturation: Enhancement of Skin Penetration and Permeation of a Lipophilic Drug. Pharm. Res. 2001, 18, 1006–1011. [Google Scholar] [CrossRef]
- Moore, J.W.; Flanner, H.H. Mathematical Comparison of Dissolution Profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Huang, J.; Wigent, R.J.; Schwartz, J.B. Drug-Polymer Interaction and Its Significance on the Physical Stability of Nifedipine Amorphous Dispersion in Microparticles of an Ammonio Methacrylate Copolymer and Ethylcellulose Binary Blend. J. Pharm. Sci. 2008, 97, 251–262. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sip, S.; Rosiak, N.; Miklaszewski, A.; Talarska, P.; Dudziec, E.; Cielecka-Piontek, J. Amorphous Form of Carvedilol Phosphate—The Case of Divergent Properties. Molecules 2021, 26, 5318. https://doi.org/10.3390/molecules26175318
Sip S, Rosiak N, Miklaszewski A, Talarska P, Dudziec E, Cielecka-Piontek J. Amorphous Form of Carvedilol Phosphate—The Case of Divergent Properties. Molecules. 2021; 26(17):5318. https://doi.org/10.3390/molecules26175318
Chicago/Turabian StyleSip, Szymon, Natalia Rosiak, Andrzej Miklaszewski, Patrycja Talarska, Ewa Dudziec, and Judyta Cielecka-Piontek. 2021. "Amorphous Form of Carvedilol Phosphate—The Case of Divergent Properties" Molecules 26, no. 17: 5318. https://doi.org/10.3390/molecules26175318
APA StyleSip, S., Rosiak, N., Miklaszewski, A., Talarska, P., Dudziec, E., & Cielecka-Piontek, J. (2021). Amorphous Form of Carvedilol Phosphate—The Case of Divergent Properties. Molecules, 26(17), 5318. https://doi.org/10.3390/molecules26175318