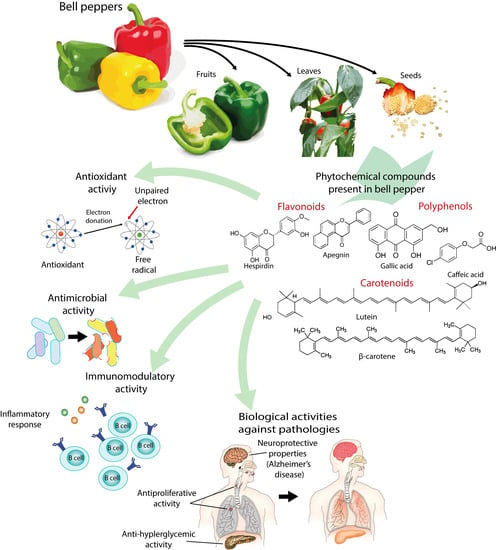
Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications
Abstract
:1. Introduction
2. Potential Food Losses and Wastes to Contribute to a Circular Economy
- (i)
- The cascading approach to use bio-based resources, including food waste.
- (ii)
- The potential for innovation in new bio-based materials, chemicals and processes contributing to the circular economy.
- (iii)
- The recycling of wood packaging and separate collection of biowaste.
3. Description of Bell Peppers
4. Phytochemicals Present in Bell Peppers
4.1. Phenols and Flavonoids
4.2. Carotenoids
4.3. Other Phytochemicals Identified in Bell Peppers Fruits, Seeds, and Leaves
5. Biological Activities of Bell Pepper Extracts
5.1. Antioxidant Activity
5.2. Antimicrobial Activity
5.3. Immunomodulatory Activity
5.4. Effect of Bell Pepper Extracts on Diverse Pathologies
5.5. Applications of Bioactive Compounds from Bell Peppers in Food Industry
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viaggi, D. The Bioeconomy: Delivering Sustainable Green Growth; CABI Publishing: Boston, MA, USA, 2018. [Google Scholar]
- Malorgio, G.; Marangon, F. Agricultural business economics: The challenge of sustainability. Agric. Food Econ. 2021, 9, 6. [Google Scholar] [CrossRef]
- Brunori, G. Biomass, Biovalue and Sustainability: Some Thoughts on the Definition of the Bioeconomy. EuroChoices 2013, 12, 48–52. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Nguyen, D.; Surendra, K.C.; Shrestha, S.; Rajendran, K.; Oechsner, H.; Xie, L.; Khanal, S.K. Anaerobic biorefinery: Current status, challenges, and opportunities. Bioresour. Technol. 2016, 215, 304–313. [Google Scholar] [CrossRef]
- Budzianowski, W.M. High-value low-volume bioproducts coupled to bioenergies with potential to enhance business development of sustainable biorefineries. Renew. Sustain. Energy Rev. 2017, 70, 793–804. [Google Scholar] [CrossRef]
- Mohan, S.V.; Butti, S.K.; Amulya, K.; Dahiya, S.; Modestra, J.A. Waste Biorefinery: A New Paradigm for a Sustainable Bioelectro Economy. Trends Biotechnol. 2016, 34, 852–855. [Google Scholar] [CrossRef]
- Smith, P.; Calvin, K.; Nkem, J.; Campbell, D.; Cherubini, F.; Grassi, G.; Korotkov, V.; Le Hoang, A.; Lwasa, S.; McElwee, P.; et al. Which practices co-deliver food security, climate change mitigation and adaptation, and combat land degradation and desertification? Glob. Chang. Biol. 2020, 26, 1532–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoma, A.; Rebecchi, S.; Bertin, L.; Fava, F. High impact biowastes from South European agro-industries as feedstock for second-generation biorefineries. Crit. Rev. Biotechnol. 2016, 36, 175–189. [Google Scholar] [CrossRef] [PubMed]
- European Commission Closing the Loop—An EU Action Plan for the Circular Economy COM/2015/0614 Final—European Environment Agency. Available online: https://www.eea.europa.eu/policy-documents/com-2015-0614-final (accessed on 26 July 2021).
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential health benefits of plant food-derived bioactive components: An overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2020; Volume 91, pp. 157–225. ISBN 9780128204702. [Google Scholar]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant secondary metabolites: An opportunity for circular economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef] [PubMed]
- FAO. World Food and Agriculture—Statistical Yearbook 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Abhishek, A.; Gupta, V.K.; Bhardwaj, M.R. Pharmacological Activity of Bell Pepper; Abhishek, A., Gupta, V.K., Bhardwaj, M.R., Eds.; JPS Scientific Publications: Tamil Nadu, India, 2021; ISBN 9788194436386. [Google Scholar]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Servicio Nacional de Inspección y Certificación de Semillas Chile (Capsicum spp.). Available online: https://www.gob.mx/snics/acciones-y-programas/chile-capsicum-spp (accessed on 17 July 2021).
- Padilha, H.K.M.; Pereira, E.D.S.; Munhoz, P.C.; Vizzotto, M.; Valgas, R.A.; Barbieri, R.L. Genetic variability for synthesis of bioactive compounds in peppers (Capsicum annuum) from Brazil. Food Sci. Technol. 2015, 35, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Ribes-Moya, A.M.; Raigón, M.D.; Moreno-Peris, E.; Fita, A.; Rodríguez-Burruezo, A. Response to organic cultivation of heirloom Capsicum peppers: Variation in the level of bioactive compounds and effect of ripening. PLoS ONE 2018, 13, e0207888. [Google Scholar] [CrossRef] [Green Version]
- Šeregelj, V.; Tumbas Šaponjac, V.; Lević, S.; Kalušević, A.; Ćetković, G.; Čanadanović-Brunet, J.; Nedović, V.; Stajčić, S.; Vulić, J.; Vidaković, A. Application of encapsulated natural bioactive compounds from red pepper waste in yogurt. J. Microencapsul. 2019, 36, 704–714. [Google Scholar] [CrossRef]
- Dagadkhair, R.A.; Shinde, D.B.; Shelke, S.A.; Kale, K.B. Vegetables & Its Health Benefits, 1st ed.; Dagadkhair, R.A., Shinde, D.B., Shelke, S.A., Kale, K.B., Eds.; Taurean Publications: Nehru Place, India, 2020; ISBN 9788194488880. [Google Scholar]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive compounds, antioxidant activity and inhibition of key enzymes relevant to Alzheimer’s disease from sweet pepper (Capsicum annuum) extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.U.E.; Taher, M.; Sanad, M.I.; Tadros, L.K. Chemical properties, phenolic profiles and antioxidant activities of pepper fruits. J. Agric. Chem. Biotechnol. 2019, 10, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Kaur, K. Preservation of sweet pepper purees: Effect on chemical, bioactive and microbial quality. J. Food Sci. Technol. 2021, 58, 3655–3660. [Google Scholar] [CrossRef]
- Rahim, R.; Mat, I. Phytochemical contents of Capsicum frutescens (Chili Padi), Capsicum annum (Chili Pepper) and Capsicum annum (Bell Peper) aqueous extracts. Int. Conf. Biol. Life Sci. 2012, 40, 164–167. [Google Scholar]
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive characteristics and antioxidant activities of nine peppers. J. Funct. Foods 2012, 4, 331–338. [Google Scholar] [CrossRef]
- Blanco-Ríos, A.K.; Medina-Juárez, L.Á.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of the phenolic and oily fractions of different sweet bell peppers. J. Mex. Chem. Soc. 2013, 57, 137–143. [Google Scholar] [CrossRef]
- USDA Peppers, Sweet, Red, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170108/nutrients (accessed on 17 July 2021).
- FAO. Save Food: Global Initiative on Food Loss and Waste Reduction; Food and Agriculture Organization United Nations: Rome, Italy, 2016; pp. 01–02. [Google Scholar]
- Almadhoun, H.R. Bell pepper Classification using Deep Learning. IJAER 2021, 5, 75–79. [Google Scholar]
- Sutliff, A.K.; Saint-cyr, M.; Hendricks, A.E.; Chen, S.S.; Doenges, K.A.; Quinn, K.; Westcott, J.; Tang, M.; Borengasser, S.J.; Reisdorph, R.M.; et al. Lipidomics-based comparison of molecular compositions of green, yellow, and red bell peppers. Metabolites 2021, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Gry, J.; Black, L.; Eriksen, F.D.; Pilegaard, K.; Plumb, J.; Rhodes, M.; Sheehan, D.; Kiely, M.; Kroon, P.A. EuroFIR-BASIS—A combined composition and biological activity database for bioactive compounds in plant-based foods. Trends Food Sci. Technol. 2007, 18, 434–444. [Google Scholar] [CrossRef]
- Devgan, K.; Kaur, P.; Kumar, N.; Kaur, A. Active modified atmosphere packaging of yellow bell pepper for retention of physico-chemical quality attributes. J. Food Sci. Technol. 2019, 56, 878–888. [Google Scholar] [CrossRef]
- Hallmann, E.; Marszałek, K.; Lipowski, J.; Jasińska, U.; Kazimierczak, R.; Średnicka-Tober, D.; Rembiałkowska, E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Guevara, L.; Domínguez-Anaya, M.Á.; Ortigosa, A.; González-Gordo, S.; Díaz, C.; Vicente, F.; Corpas, F.J.; Pérez Del Palacio, J.; Palma, J.M. Identification of compounds with potential therapeutic uses from sweet pepper (Capsicum annuum L.) fruits and their modulation by nitric oxide (no). Int. J. Mol. Sci. 2021, 22, 4476. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, H.W.; Lee, M.K.; Kim, H.J.; Kim, J.B.; Choe, J.S.; Lee, Y.M.; Jang, H.H. Antioxidant and anti-inflammatory activities in relation to the flavonoids composition of pepper (Capsicum annuum L.). Antioxidants 2020, 9, 986. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Faloye, Y.M. Effect of combination on the antioxidant and inhibitory properties of tropical pepper varieties against α-amylase and α-gflucosidase activities in vitro. J. Med. Food 2011, 14, 1152–1158. [Google Scholar] [CrossRef]
- González-García, Y.; Cárdenas-álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-De-la-fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of three nanoparticles (Se, Si and Cu) on the bioactive compounds of bell pepper fruits under saline stress. Plants 2021, 10, 217. [Google Scholar] [CrossRef]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef] [Green Version]
- Gómez-García, M.D.R.; Ochoa-Alejo, N. Biochemistry and molecular biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Saucedo, A.; Barrera-Necha, L.L.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Hernández-López, M. Extension of the postharvest quality of bell pepper by applying nanostructured coatings of chitosan with Byrsonima crassifolia extract (L.) Kunth. Postharvest Biol. Technol. 2019, 149, 74–82. [Google Scholar] [CrossRef]
- Marín, A.; Gil, M.I.; Flores, P.; Hellín, P.; Selma, M.V. Microbial quality and bioactive constituents of sweet peppers from sustainable production systems. J. Agric. Food Chem. 2008, 56, 11334–11341. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Martínez-Guirado, C.; Del Mar Rebolloso-Fuentes, M.; Carrique-Pérez, A. Nutrient composition and antioxidant activity of 10 pepper (Capsicum annuun) varieties. Eur. Food Res. Technol. 2006, 224, 1–9. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; Sánchez, E.; Carvajal-Millán, E.; Munoz-Márquez, E.; Guevara-Aguilar, A. Characterization of the nutraceutical quality and antioxidant activity in bell pepper in response to grafting. Molecules 2013, 18, 15689–15703. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Adami, E.R.; Corso, C.R.; Turin-Oliveira, N.M.; Galindo, C.M.; Milani, L.; Stipp, M.C.; do Nascimento, G.E.; Chequin, A.; da Silva, L.M.; de Andrade, S.F.; et al. Antineoplastic effect of pectic polysaccharides from green sweet pepper (Capsicum annuum) on mammary tumor cells in vivo and in vitro. Carbohydr. Polym. 2018, 201, 280–292. [Google Scholar] [CrossRef]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Pérez-Morales, R.; Ortiz, J.C.R.; García-Hernández, J.L. Characterization of different Capsicum varieties by evaluation of their capsaicinoids content by high performance liquid chromatography, determination of pungency and effect of high temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Azevedo, J.; Pereira, M.J.; Valentão, P.; Andrade, P.B. Chemical assessment and antioxidant capacity of pepper (Capsicum annuum L.) seeds. Food Chem. Toxicol. 2013, 53, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Games, P.D.; da Silva, E.Q.G.; de Oliveira Barbosa, M.; Almeida-Souza, H.O.; Fontes, P.P.; de Magalhães, M.J., Jr.; Pereira, P.R.G.; Prates, M.V.; Franco, G.R.; Faria-Campos, A.; et al. Computer aided identification of a Hevein-like antimicrobial peptide of bell pepper leaves for biotechnological use. BMC Genom. 2016, 17, 999. [Google Scholar] [CrossRef] [Green Version]
- Games, P.D.; Koscky-Paier, C.R.; Almeida-Souza, H.O.; Barbosa, M.O.; Antunes, P.W.P.; Carrijo, L.C.; Pereira, P.R.G.; Baracat-Pereira, M.C. In vitro anti-bacterial and anti-fungal activities of hydrophilic plant defence compounds obtained from the leaves of bell pepper (Capsicum annuum L.). J. Hortic. Sci. Biotechnol. 2013, 88, 551–558. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; Sanchez, E.; Muñoz-Marquez, E.; Sida-Arreola, J.P.; Flores-Cordova, M.A. Bioactive compounds and antioxidant activity in different grafted varieties of bell pepper. Antioxidants 2015, 4, 427–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Jeon, G.I.; Kim, J.M.; Park, E. Antioxidant activity and antiproliferative action of methanol extracts of 4 different colored bell peppers (Capsicum annuum L.). Food Sci. Biotechnol. 2012, 21, 543–550. [Google Scholar] [CrossRef]
- Norafida, A.; Aminah, A. Effect of blanching treatments on antioxidant activity of frozen green Capsicum (Capsicum annuum L. var. bell pepper). Int. Food Res. J. 2018, 25, 1427–1434. [Google Scholar]
- Sora, G.T.S.; Haminiuk, C.W.I.; da Silva, M.V.; Zielinski, A.A.F.; Gonçalves, G.A.; Bracht, A.; Peralta, R.M. A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: An application of chemometrics. J. Food Sci. Technol. 2015, 52, 8086–8094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdizadeh Shotorbani, N.; Jamei, R.; Heidari, R. Antioxidant activities of two sweet pepper Capsicum annuum L. varieties phenolic extracts and the effects of thermal treatment. Avicenna J. Phytomed. 2013, 3, 25–34. [Google Scholar] [CrossRef]
- Qiao, G.H.; Wexxin, D.; Zhigang, X.; Sami, R.; Khojah, E.; Amanullah, S. Antioxidant and anti-inflammatory capacities of pepper tissues. Ital. J. Food Sci. 2020, 32, 265–274. [Google Scholar] [CrossRef]
- Cortés-Estrada, C.E.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Castañeda-Pérez, E.; Meza-Márquez, O.G.; del Socorro López-Cortez, M.; Hernández-Martínez, D.M. Prediction of total phenolics, ascorbic acid, antioxidant capacities, and total soluble solids of Capsicum annuum L. (bell pepper) juice by FT-MIR and multivariate analysis. LWT 2020, 126, 109285. [Google Scholar] [CrossRef]
- Ilic, Z.S.; Mirecki, N.; Fallik, E. Cultivars differences in keeping quality and bioactive constituents. J. Adv. Biotechnol. 2014, 4, 313–318. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M. Dehydration of red bell-pepper (Capsicum annuum L.): Change in drying behavior, colour and antioxidant content. Food Bioprod. Process. 2011, 89, 504–513. [Google Scholar] [CrossRef]
- Prabakaran, S.; Ramu, L.; Veerappan, S.; Pemiah, B.; Kannappan, N. Effect of different solvents on volatile and non-volatile constituents of red bell pepper (Capsicum annuum L.) and their in vitro antioxidant activity. J. Food Meas. Charact. 2017, 11, 1531–1541. [Google Scholar] [CrossRef]
- Chuah, A.M.; Lee, Y.C.; Yamaguchi, T.; Takamura, H.; Yin, L.J.; Matoba, T. Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. 2008, 111, 20–28. [Google Scholar] [CrossRef]
- Bae, H.; Jayaprakasha, G.K.; Crosby, K.; Jifon, J.L.; Patil, B.S. Influence of extraction solvents on antioxidant activity and the content of bioactive compounds in non-pungent peppers. Plant Foods Hum. Nutr. 2012, 67, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mier, L.; Jimenez-Garcia, S.N.; Guevara-González, R.G.; Feregrino-Perez, A.A.; Contreras-Medina, L.M.; Torres-Pacheco, I. Elicitor mixtures significantly increase bioactive compounds, antioxidant activity, and quality parameters in sweet bell pepper. J. Chem. 2015, 2015, 269296. [Google Scholar] [CrossRef] [Green Version]
- Dorantes, L.; Colmenero, R.; Hernandez, H.; Mota, L.; Jaramillo, M.E.; Fernandez, E.; Solano, C. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. Int. J. Food Microbiol. 2000, 57, 125–128. [Google Scholar] [CrossRef]
- Hu, X.; Saravanakumar, K.; Jin, T.; Wang, M.H. Effects of yellow and red bell pepper (paprika) extracts on pathogenic microorganisms, cancerous cells and inhibition of survivin. J. Food Sci. Technol. 2021, 58, 1499–1510. [Google Scholar] [CrossRef]
- López-Muñoz, N.R.; Romero-Bastidas, M.; Arce-Amézquita, P.M.; Hernández-Rubio, J.S. Antifungal activity of antioxidants derived from four cultivars of Capsicum spp. against phytopathogenic fungi. Ecosistemas Recur. Agropecu. 2019, 6, 487–498. [Google Scholar] [CrossRef]
- da Silva França, K.R.; Paiva, Y.F.; de Azevedo, P.T.M.; da Nóbrega, L.P.; da Silva, E.V.; Cardoso, T.A.L. Extratos de pimentão vermelho (Capsicum annuum) sobre Colletotrichum gloeosporioides in vitro. Rev. Verde Agroecol. Desenvolv. Sustentável 2019, 14, 382–388. [Google Scholar] [CrossRef]
- Careaga, M.; Fernández, E.; Dorantes, L.; Mota, L.; Jaramillo, M.E.; Hernandez-Sanchez, H. Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int. J. Food Microbiol. 2003, 83, 331–335. [Google Scholar] [CrossRef]
- Aljaloud, S.O.; Gyawali, R.; Reddy, M.R.; Ibrahim, S.A. Antibacterial activity of red bell pepper against Escherichia coli O157:H7 in ground beef. Internet J. Food Saf. 2012, 14, 44–47. [Google Scholar]
- Mokhtar, M.; Ginestra, G.; Youcefi, F.; Filocamo, A.; Bisignano, C.; Riazi, A. Antimicrobial activity of selected polyphenols and capsaicinoids identified in pepper (Capsicum annuum L.) and their possible mode of interaction. Curr. Microbiol. 2017, 74, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Sancho, R.; Lucena, C.; Macho, A.; Calzado, M.A.; Blanco-Molina, M.; Minassi, A.; Appendino, G.; Muñoz, E. Immunosuppressive activity of capsaicinoids: Capsiate derived from sweet peppers inhibits NF-κB activation and is a potent antiinflammatory compound in vivo. Eur. J. Immunol. 2002, 32, 1753–1763. [Google Scholar] [CrossRef]
- Hazekawa, M.; Hideshima, Y.; Ono, K.; Nishinakagawa, T.; Kawakubo-Yasukochi, T.; Takatani-Nakase, T.; Nakashima, M. Anti-inflammatory effects of water extract from bell pepper (Capsicum annuum L. var. grossum) leaves in vitro. Exp. Ther. Med. 2017, 14, 4349–4355. [Google Scholar] [CrossRef]
- Popov, S.V.; Ovodova, R.G.; Golovchenko, V.V.; Popova, G.Y.; Viatyasev, F.V.; Shashkov, A.S.; Ovodov, Y.S. Chemical composition and anti-inflammatory activity of a pectic polysaccharide isolated from sweet pepper using a simulated gastric medium. Food Chem. 2011, 124, 309–315. [Google Scholar] [CrossRef]
- Sarker, M.R.; Gohda, E. Promotion of anti-keyhole limpet hemocyanin IgM and IgG antibody productions in vitro by red bell pepper extract. J. Funct. Foods 2013, 5, 1918–1926. [Google Scholar] [CrossRef]
- Sarker, M.R. Evaluation of red and green colored bell peppers for the production of polyclonal IgM and IgG antibodies in murine spleen cells. Bangladesh Pharm. J. 2021, 24, 45–53. [Google Scholar] [CrossRef]
- Goto, T.; Sarker, M.R.; Zhong, M.; Tanaka, S.; Gohda, E. Enhancement of immunoglobulin M production in B cells by the extract of red bell pepper. J. Health Sci. 2010, 56, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Kim, R.Y.; Park, E. Antidiabetic activity of fruits and vegetables commonly consumed in Korea: Inhibitory potential against α-glucosidase and insulin-like action in vitro. Food Sci. Biotechnol. 2012, 21, 1187–1193. [Google Scholar] [CrossRef]
- Nagasukeerthi, P.; Mooventhan, A.; Manjunath, N.K. Short-term effect of add on bell pepper (Capsicum annuum var. grossum) juice with integrated approach of yoga therapy on blood glucose levels and cardiovascular functions in patients with type 2 diabetes mellitus: A randomized controlled study. Complement. Ther. Med. 2017, 34, 42–45. [Google Scholar] [CrossRef]
- Fuentes, A.L.; Hennessy, K.; Pascual, J.; Pepe, N.; Wang, I.; Santiago, A.; Chaggan, C.; Martinez, J.; Rivera, E.; Cota, P.; et al. Identification of plant extracts that inhibit the formation of diabetes-linked IAPP amyloid. J. Herb. Med. 2016, 6, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Obaidi, W.M.L. Study of synergistic inhibitory effectiveness of mixed of sweet bell pepper extract and virgin olive oil in α-amylase and α-glucosidase activity in serum of male rats infected with experimental diabetes. Tikrit J. Pure Sci. 2015, 20, 90–96. [Google Scholar]
- Shukla, S.; Kumar, D.A.; Anusha, S.V.; Tiwari, A.K. Antihyperglucolipidaemic and anticarbonyl stress properties in green, yellow and red sweet bell peppers (Capsicum annuum L.). Nat. Prod. Res. 2016, 30, 583–589. [Google Scholar] [CrossRef] [PubMed]
- El Hamss, R.; Idaomar, M.; Alonso-Moraga, A.; Muñoz Serrano, A. Antimutagenic properties of bell and black peppers. Food Chem. Toxicol. 2003, 41, 41–47. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Jin, J.S.; Cho, Y.A.; Lee, J.H.; Park, S.; Jeong, S.W.; Kim, Y.H.; Lim, C.S.; Abd El-Aty, A.M.; Kim, G.S.; et al. Determination of polyphenols in three Capsicum annuum L. (bell pepper) varieties using high-performance liquid chromatographytandem mass spectrometry: Their contribution to overall antioxidant and anticancer activity. J. Sep. Sci. 2011, 34, 2967–2974. [Google Scholar] [CrossRef]
- Ogunruku, O.O.; Oboh, G.; Passamonti, S.; Tramer, F.; Boligon, A.A. Capsicum annuum var. grossum (Bell Pepper) inhibits β-secretase activity and β-amyloid1-40 aggregation. J. Med. Food 2017, 20, 124–130. [Google Scholar] [CrossRef]
- Toda, T.; Sunagawa, T.; Kanda, T.; Tagashira, M.; Shirasawa, T.; Shimizu, T. Apple procyanidins suppress amyloid β-protein aggregation. Biochem. Res. Int. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Mendes Gomes, L.M.; Petito, N.; Costa, V.G.; Falcão, D.Q.; De Lima Araújo, K.G. Inclusion complexes of red bell pepper pigments with β-cyclodextrin: Preparation, characterisation and application as natural colorant in yogurt. Food Chem. 2014, 148, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Lobo, F.A.T.F.; Silva, V.; Domingues, J.; Rodrigues, S.; Costa, V.; Falcão, D.; de Lima Araújo, K.G. Inclusion complexes of yellow bell pepper pigments with β-cyclodextrin: Preparation, characterisation and application as food natural colorant. J. Sci. Food Agric. 2018, 98, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Uarrota, V.G.; Maraschin, M.; de Bairros, Â.D.F.M.; Pedreschi, R. Factors affecting the capsaicinoid profile of hot peppers and biological activity of their non-pungent analogs (Capsinoids) present in sweet peppers. Crit. Rev. Food Sci. Nutr. 2021, 61, 649–665. [Google Scholar] [CrossRef] [PubMed]
| Component | Value |
|---|---|
| Energy (Kcal/KJ) | 26/111 |
| Moisture (g) | 92.2 |
| Carbohydrates (g) | 6.03 |
| Dietary fiber (g) | 2.1 |
| Protein (g) | 0.99 |
| Total fat (g) | 0.30 |
| Ash (g) | 0.47 |
| Vitamins | |
| Niacin (mg) | 0.979 |
| Pyridoxine (mg) | 0.291 |
| Vitamin A (IU) | 3131 |
| Vitamin C (mg) | 127.7 |
| Vitamin E (mg) | 1.58 |
| Vitamin K (µg) | 4.9 |
| Minerals | |
| Sodium (mg) | 4 |
| Potassium (mg) | 211 |
| Calcium (mg) | 7 |
| Magnesium (mg) | 12 |
| Phosphorus (mg) | 26 |
| Bioactive Compound | Bell Pepper Color | Ref. | |||
|---|---|---|---|---|---|
| Polyphenols | Green | Red | Yellow | Orange | |
| Total polyphenols (GAE mg/g) | 4.51–52.65 | 7.86–42.57 | 7.44–43.59 | 12.35 | [22,23,24,25,26,27] |
| 3,4-Dihydroxybenzoic acid (µg/g) | 0.40 | [26] | |||
| 3,4,5-methoxy-cinnamic acid (µg/g) | 14.69 | 13.82 | 13.61 | [23] | |
| 4-Aminobenzoic acid (µg/g) | 22.09 | 21.34 | 50.19 | [23] | |
| α-coumaric acid (µg/g) | 3.36 | 7.65 | 6.41 | [23] | |
| Benzoic acid (µg/g) | 66.55 | 23.17–111.81 | 173.04 | [23,26] | |
| Catechol (µg/g) | 279.42 | 89.77 | 225.73 | [23] | |
| Chlorogenic acid (µg/g) | 60.84–290.08 | 60.47–221.53 | 103.78–136.51 | 117.54 | [23,27] |
| Cinnamic acid (µg/g) | 3.51 | 8.11 | 4.65 | [23] | |
| Gallic acid (µg/g) | 89.98 | 115.74 | 119.48 | 900 | [23,35] |
| Caffeic acid (µg/g) | 18.09-108.82 | 41.33-67.78 | 52.42–62.96 | 38.03 | [23,27] |
| Ellagic acid (µg/g) | 106.67 | 172.18 | 144.52 | [23] | |
| Ferulic acid (µg/g) | 23.59–48.42 | 11.88–27.67 | 24.75–35.14 | 13.45 | [22,23] |
| Myricetin (µg/g) | 658.19 | 244.33 | 151.35 | 100.62 | [27] |
| P-Coumaric acid (µg/g) | 19.62–46.69 | 9.96–26.07 | 18.14–24.75 | 13.45 | [22,23] |
| P-OH-benzoic acid (µg/g) | 65.85 | 395.16 | 123.19 | [23] | |
| Protocatechuic acid (µg/g) | 116.09 | 97.21 | 95.37 | [23] | |
| Pyrogallol (µg/g) | 572.77 | 757.66 | 2175.89 | [23] | |
| Resveratrol (µg/g) | 174.34 | 111.57 | 90.78 | 89.72 | [27] |
| Rosmarinic acid (µg/g) | 120 | [20] | |||
| Sinapinic acid (µg/g) | 117 | [20] | |||
| Vanillic acid (µg/g) | 43.85 | 11–17.70 | 31.62 | [23,26] | |
| Vanillin (µg/g) | 0.11 | [26] | |||
| Flavonoids | |||||
| Total flavonoids (QE mg/g) | 2.1–41 | 3.5–39 | 2.4–33 | 12.35 | [24,27,34] |
| Apig. 6-arbinose 8-galactose (µg/g) | 151.66 | 156.42 | 67.88 | [23,24] | |
| Apig. 6-rhamnose 8-glucose (µg/g) | 170.96 | 314.70 | 77.31 | [23] | |
| Apigenin. 7-O-neohespiroside (µg/g) | 33.55 | 40.27 | 4.51 | [23] | |
| Apegnin (µg/g) | 2.12 | 36.28 | 1.54 | [23] | |
| Catechin (µg/g) | 295.39 | 793.50 | 745.53 | 4.81 | [23] |
| Epicatechin (µg/g) | 505 | [20] | |||
| Hespirtin (µg/g) | 38.05 | 37.00 | 7.07 | [23] | |
| Hespirdin (µg/g) | 1065.65 | 1513.13 | 213.06 | [23] | |
| Kampferol (µg/g) | 22.48 | 31.15 | 9.53 | [23] | |
| Luteolin 7-glucose (µg/g) | 181.12 | 413.57 | 92.21 | [23] | |
| Luteolin (µg/g dw) | 62.31 | 68.43 | 95.89 | 56.34–154.03 | [22,27] |
| Naringenin (µg/g) | 13.64 | 1.54 | 2.12 | [23] | |
| Naringin (µg/g) | 275.00 | 50.13 | 190.19 | [23] | |
| Quercetin (µg/g) | 16.24–71.71 | 46.36–91.98 | 9.66–102.33 | 92 | [22,23] |
| Quercetrin (µg/g) | 394.23 | 9.97–241.83 | 62.34 | 42.87 | [23,27,35] |
| Rutin (µg/g) | 93.43 | 290.39 | 49.51 | [23] | |
| Carotenoids | Bell Pepper Color | Ref. | |||
|---|---|---|---|---|---|
| Green | Red | Yellow | Orange | ||
| Total carotenoids (µg/g) | 1219–1513.5 | 7137–8800 | 2236.3–2834 | 5292 | [23,26,27,42] |
| 5,6,-epoxide capsanthin (µg/g) | 513 | [43] | |||
| α-carotene (µg/g) | 3.56 | 4.22–21.27 | 9.02 | [22,44] | |
| β-carotene (µg/g) | 1.86–12.2 | 0.70–43.9 | 3.86–15.9 | 56.6 | [27,34,44] |
| 13-Cis-β-carotene (µg/g) | 10.7 | 36 | 12 | [45] | |
| 9-Cis-β-carotene (µg/g) | 12.9 | 38 | 3.2 | [45] | |
| 9,13-Cis-β-carotene (µg/g) | 11.6 | 139 | 12.5 | [45] | |
| β-Zea-carotene (µg/g) | 97.3 | 4.6 | [45] | ||
| α-cryptoxanthin (µg/g) | 27 | 0.9–27 | 0.3 | [34,45] | |
| β-cryptoxanthin (µg/g) | 4 | 40.49 | 7.55–19.5 | 19.45 | [22,44] |
| Cis-β-cryptoxanthin (µg/g) | 20 | 20 | 1.1 | 0.3 | [44] |
| Antheraxanthin (µg/g) | 44 | [43] | |||
| Capsanthin (µg/g) | 16.13 | 178.20 | 45.48 | 45.48 | [22] |
| Capsorubin (µg/g) | 1.4–48 | [34,43] | |||
| Cis-beta-carotene (µg/g) | 9.64 | 34.28 | 6.81 | 8.32 | [22] |
| Cis-capsanthin | 3.8 | [43] | |||
| Cis-zexanthin (µg/g) | 1.5 | [34] | |||
| Chlorophyll (µg/g) | 150.8 | 52.3 | 61.4 | [24] | |
| Cryptoxanthin (µg/g) | 3.2 | [34] | |||
| Cryptoflavin (µg/g) | 2.1 | [34] | |||
| Cucurbitaxanthin (µg/g) | 81 | [43] | |||
| Lycopene (µg/g) | 4.8–322 | 2.5 | [46,47] | ||
| Lutein (µg/g) | 60.04–76.5 | 95.5–115.16 | 45.16 | [22,44] | |
| 13-Cis-luteion | 3 | 12 | [45] | ||
| All-trans-lutein | 14 | 37 | 58 | [45] | |
| Mutatoxanthin (µg/g) | 49 | [43] | |||
| Neoxanthin (µg/g) | 190 | [43] | |||
| Retinol (RE µg/g) | 0.313 | 1.57 | [44] | ||
| Trans-β-Carotene (µg/g) | 13.09 | 41.72 | 6.81 | 8.32 | [22] |
| Violaxanthin (µg/g) | 12 | 48 | [43] | ||
| Cis-violaxanthin (µg/g) | 0.5 | 174 | 1.8 | [45] | |
| Zeaxanthin (µg/g) | 35 | 8.8–70.71 | 48.3 | 191.76 | [22,27,44] |
| Cis-zeaxanthin (µg/g) | 24 | [45] | |||
| Bell Pepper Color | Source | Bioactive Extracts or Compounds | Method of Antioxidant Capacity | Ref. | ||
|---|---|---|---|---|---|---|
| ABTS• (µmol TE/g) | DPPH• (%) | FRAP (µg TE/g) | ||||
| Green | Fruit pulp | Ethanolic extract | 78 | [54] | ||
| Ethanolic extract | 40 | [45] | ||||
| Methanolic extract | 630 | * 1153 | [55] | |||
| Methanolic extract | 80 | 1400 | [56] | |||
| Ethanolic extract | 17.17 | ¥ 2.28 | ¥ 3.99 | [57] | ||
| Ethanolic extract | ¥ 25.15 | 30.15 | [22] | |||
| Methanolic extract | * 1114 | [23] | ||||
| Methanolic extract | 90 | [58] | ||||
| NI | 105 | ¥ 70 | [27] | |||
| Methanolic extract | 4 | ¥ 30 | 46 | [59] | ||
| Fruit juice | Direct extraction | 8.64 | ¥ 0.86 | [60] | ||
| Seeds | Ethanolic extract | 89.25 | ¥ 11.32 | ¥ 9.94 | [57] | |
| Aqueous extract | 0.413 | [51] | ||||
| Red | Fruit pulp | Ethanolic extract | 79.65 | [54] | ||
| Methanolic extract | 54–70 | [42] | ||||
| Ethanolic extract | 80 | [46] | ||||
| Lipophilic fraction | 4.05 | [61] | ||||
| Ethanolic extract | 50 | [45] | ||||
| Methanolic extract | 800 | * 882 | [55] | |||
| Methanolic extract | 18 | [35] | ||||
| Ethanolic extract | ¥ 23.79 | 28.12 | [22] | |||
| Methanolic extract | * 1832 | [23] | ||||
| Aqueous extract | * 366 | * 125 | [26] | |||
| Methanolic extract | 80 | [58] | ||||
| NI | 90 | ¥ 80 | [27] | |||
| Methanolic extract | 55.64 | 76 | [62] | |||
| Ethanolic extract | 50 | [63] | ||||
| Methanolic extract | 4 | ¥ 25 | 39 | [59] | ||
| Methanolic extract | ¥ 10 | [64] | ||||
| Fruit juice | Direct extraction | 14.02 | ¥ 1.05 | [60] | ||
| Orange | Fruit pulp | Ethanolic extract | 70 | [54] | ||
| Lipophilic fraction | 5.20 | [61] | ||||
| Ethanolic extract | 75 | [45] | ||||
| Methanolic extract | 880 | * 694 | [55] | |||
| Methanolic extract | 21 | [35] | ||||
| Ethanolic extract | ¥ 22.20 | 25.20 | [30] | |||
| NI | 85 | ¥ 50 | [27] | |||
| Fruit juice | Direct extraction | 13.66 | ¥ 1.12 | [60] | ||
| Yellow | Fruit pulp | Lipophilic fraction | 3.33 | [61] | ||
| Ethanolic extract | 64.90 | [54] | ||||
| Ethanolic extract | 70 | [46] | ||||
| Ethanolic extract | 80 | [45] | ||||
| Methanolic extract | 790 | * 811 | [55] | |||
| Methanolic extract | 22 | [35] | ||||
| Aqueous ethanol extarct | ¥ 18.23 | 19.87 | [22] | |||
| Methanolic extract | * 3267 | [23] | ||||
| NI | 65 | ¥ 60 | [27] | |||
| Methanolic extract | 5 | ¥ 35 | 40 | [59] | ||
| Purple | Fruit pulp | Methanolic extract | 20 | [35] | ||
| Dark violet | Fruit pulp | Methanolic extract | 15 | [35] | ||
| Bell Pepper Color | Source | Bioactive Extracts or Compounds | Dose | Model Assay | Effect | Ref. |
|---|---|---|---|---|---|---|
| NI | Fruit pulp | Isopropanol extract | 20 µL of extract | DDA against L. monocytogenes, S. typhymurium, B. cereus, and S. aureus | Extract showed inhibition of growth in four bacteria. | [67] |
| Red | Fruit pulp | Methanolic extract | 20 µg/mL | DDA against B. cereus, E. coli, S. aureus, and P. aeruginosa | Strain-dependent antimicrobial effect. | [68] |
| Yellow | Fruit pulp | Methanolic extract | 20 mg/mL | B. cereus, E. coli, S. aureus, and P. aeruginosa | Strain-dependent antimicrobial effect. | [68] |
| Red | Fruit pulp | Ethanolic extract | 300 mg/L | F. andiyasi and Cochliobolus spp. | Extracts showed fungistatic effect | [69] |
| Red | Fruit pulp | Ethanolic extract | 5% v/v | Cholletotrichum gloeosporioides | Extracts showed antifungal effect. | [70] |
| NI | Fruit pulp | Ethanolic extract | 1.5 mg/100 g | S. typhimurium and P. aureginosa | Extract showed bactericidal effect against pathogenic bacteria | [71] |
| NI | Fruit pulp | NI | 1000 µL of crude extract | DDA against Escherichia coli O157H:7 | Extract exhibited antimicrobial effect in a dose-dependent manner. | [72] |
| NI | Fruit pulp | NI | DDA against S. aureus, L. monocytogenes, S. typhimurium, E. coli, B. subtilis, P. mirabilis, L. acidophilus, and L. plantarum | Extract showed antimicrobial effect against pathogenic bacteria, while in lactic acid bacteria exhibited a prebiotic-like effect. | [73] | |
| Leaves | Peptide | NI | DDA against Clavibacter michiganensis spp. michiganensis, Ralstonia solanacearum, Pseudomona syringae pv. tomato, Xanthomonas axonopodis pv. phaseoli, and Erwinia carotovora sp. carotovora | Antimicrobial effect was seen in a strain- and concentration-dependent response. | [53] | |
| Leaves | Peptide | NI | DDA against Clavibacter michiganensis spp. michiganensis and Ralstonia solanacearum | Peptide exhibited an effect on microbial growth. | [52] |
| Color of Bell Pepper | Source | Bioactive Extracts or Compounds | Dose | Model Assay | Effect | Ref. |
|---|---|---|---|---|---|---|
| NI | NI | Nor-dihydrocapsiate | NI | Bioassay in Jurkat cells and BALB/c mice model | Compound exhibited immunosuppressive effects in a dose-dependent response via the inhibition of NF- kBis by phosphorylation of MAPK p38. | [74] |
| Leaves | Aqueous extract | 5 µg/mL | In vitro on mouse spleen cells | Aqueous extract had anti-inflammatory effects mediated by suppression of the T-cell activation. | [75] | |
| Red | Pulp powder | Pectin polysaccharide isolated | 40–100 mg/kg | In vivo in male BALB/c mice model | Extract decreased TNF release. | [76] |
| Red | Pulp powder | Aqueous extract | 0.75 and 1.5 mg/mL | In vitro murine splenocytes and B cells | The extract promoted the production of IgM and IgG. | [77] |
| Red | Pulp powder | Aqueous extract | 0.375, 0.75, 1.5, 2.25 mg/mL | In vitro and Ex vivo murine splenocytes and B cells | Aqueous extract promoted the production of both IgM and IgG antibodies in polyclonal response. | [78] |
| Red | Fruit pulp | Aqueous extract | 1.5 mg/mL | In vitro and Ex vivo murine splenocytes and B cells | The extract dose-dependently increased polyclonal IgM production. | [79] |
| Pathology | Bell Pepper Color | Source | Bioactive Extracts or Compounds | Dose | Model Assay | Effect | Ref. |
|---|---|---|---|---|---|---|---|
| Diabetes | Green | Fruit juice | Whole juice/ethanol extracts | 50 mg/mL | α-glucosidase inhibitory activity | Extract exhibited α-glucosidase inhibitory effects. | [80] |
| Green | Fruit juice | Ethanol extracts | 100 µg/mL | growth of 3T3-L1 preadipocytes | Extract increased the survive rate of preadipocyte cells | [80] | |
| Green | Fruit juice | Ethanol extracts | 100 µg/mL | 3T3-L1 differentiation into adipocytes induced | Extracts promote the 3T3-L1 cells differentiation rate | [80] | |
| NI | Fruit juice | Fruit juice | 100 mL/twice a day | Randomized controlled study in humans | Fruit juice reduces post-prandial blood glucose and blood pressure. | [81] | |
| Red | Fruit pulp | Ethyl acetate extracts | 20 µL | In vitro in HeLa cells | Extract inhibited the protein islet amyloid polypeptide. | [82] | |
| Red | Fruit pulp | Extract mixed with virgin olive oil | 2 to 8 mL/kg body weight | In vivo animal assay in adult male rats | The mixture inhibited amylase and α-glucosidase activity. | [83] | |
| Green Red Yellow | NI | methanol extract | NI | NI | Extracts had α-glucosidase-inhibitory effects. | [84] | |
| Cancer | NI | Powdered | Aqueous extracts | 10% v/w | animal assay with Drosophila larval (SMART assay) | Aqueous extracts showed antimutagenic activity. | [85] |
| Red | Fruit pulp | Methanol extract | 125 µg/mL | In vitro in NIH3T3 and A549 cells | The extract exhibited strong cytotoxicity in A549 cells. | [68] | |
| Yellow | Fruit pulp | Methanol extract | 125 µg/mL | In vitro in NIH3T3 and A549 cells | Selective cytotoxic activity against A549 cells. | [68] | |
| Green | Fruit pulp | Pectic polysaccharides | 150 mg/kg | In vivo animal model in Ehrlich tumor-bearing mice | Significantly reduced tumor growth. | [49] | |
| Green | Fruit pulp | Pectic polysaccharides | 0.1 mg/mL | In vitro in lineages of human mammary cancer cells (MCF-7, MDA-MB-231, and MDA-MB-436) | Selective cytotoxic activity against MCF-7, MDA-MB-231, and MDA-MB-436 cell lines. | [49] | |
| Green Yellow Red | Fruit pulp | Polyphenol mixtures | 1.2 mg/L | In vitro in human gastric adenocarcinoma cells, A549 human lung carcinoma cells, and HeLa human cervical carcinoma cells | Extracts showed cytotoxic effects against all cancer cell lines in a dose-dependent response. | [86] | |
| Alzheimer’s disease | NI | Powdered | Aqueous extracts / E-capsiate, Z-capsiate, dihydrocapsiate and nor-dihydrocapsiate, | 1–10 g/L | In vitro Peptides agregation test | Bell pepper extracts were able to inhibit b- secretase activity and aggregation of Ab1–40 peptides | [87] |
| Application | Color | Source | Bioactive Extracts or Compounds | Dose | Model Assay | Effect | Ref. |
|---|---|---|---|---|---|---|---|
| Food preservation | NI | Fruit pulp | Ethanolic extract | 1.5 mg/100 g | Raw beef meat | Extract help to extend the shelf life of beef meat. | [71] |
| NI | Fruit pulp | NI | 5% v/v | Grounded meat | Extract help to extend the shelf life of ground meat. | [72] | |
| Natural colorant | Red | Fruit pulp | Ethanolic extract | NI | Yogurt | Capsules improve color of yogurt. | [89] |
| Red | Fruit pulp | Ethanolic extract | 10% w/v | Yogurt | Capsules improve sensory attributes of yogurt. | [20] | |
| Yellow | Fruit pulp | Ethanolic extract | 0.6 g/L | Isotonic drink | Compounds improve the drink color. | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya-Esparza, L.M.; Mora, Z.V.-d.l.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26, 5341. https://doi.org/10.3390/molecules26175341
Anaya-Esparza LM, Mora ZV-dl, Vázquez-Paulino O, Ascencio F, Villarruel-López A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules. 2021; 26(17):5341. https://doi.org/10.3390/molecules26175341
Chicago/Turabian StyleAnaya-Esparza, Luis Miguel, Zuamí Villagrán-de la Mora, Olga Vázquez-Paulino, Felipe Ascencio, and Angélica Villarruel-López. 2021. "Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications" Molecules 26, no. 17: 5341. https://doi.org/10.3390/molecules26175341
APA StyleAnaya-Esparza, L. M., Mora, Z. V. -d. l., Vázquez-Paulino, O., Ascencio, F., & Villarruel-López, A. (2021). Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules, 26(17), 5341. https://doi.org/10.3390/molecules26175341