Synthesis, Crystal Structure, and Luminescence of Cadmium(II) and Silver(I) Coordination Polymers Based on 1,3-Bis(1,2,4-triazol-1-yl)adamantane
Abstract
:1. Introduction
2. Results and Discussion
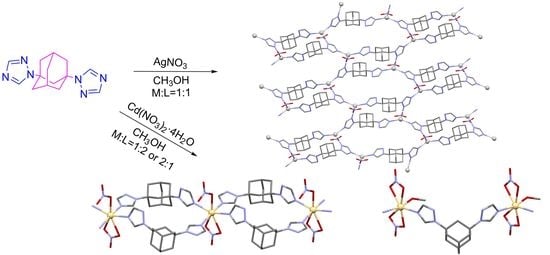
2.1. Synthesis of the Coordination Polymers
2.2. Crystal Structures of the Coordination Polymers
2.3. Powder X-ray Diffraction, Thermal Analysis, and FT-IR Spectroscopy
2.4. Luminescent Properties of the Coordination Polymers
3. Materials and Methods
3.1. Synthesis of the Coordination Polymers
3.2. Characterization Methods
3.3. X-ray Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Slyusarchuk, V.D.; Kruger, P.E.; Hawes, C.S. Cyclic Aliphatic Hydrocarbons as Linkers in Metal-Organic Frameworks: New Frontiers for Ligand Design. ChemPlusChem 2020, 85, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, H.; Hierso, J.-C. Porous Materials Based on 3-Dimensional Td-Directing Functionalized Adamantane Scaffolds and Applied as Recyclable Catalysts. Chem. Mater. 2019, 31, 619–642. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological profile of natural and synthetic compounds with rigid adamantane-based scaffolds as potential agents for the treatment of neurodegenerative diseases. Biochem. Biophys. Res. Commun. 2020, 529, 1225–1241. [Google Scholar] [CrossRef] [PubMed]
- Spilovska, K.; Zemek, F.; Korabecny, J.; Nepovimova, E.; Soukup, O.; Windisch, M.; Kuca, K. Adamantane—A Lead Structure for Drugs in Clinical Practice. Curr. Med. Chem. 2016, 23, 3245–3266. [Google Scholar] [CrossRef]
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef]
- Štimac, A.; Šekutor, M.; Mlinarić-Majerski, K.; Frkanec, L.; Frkanec, R. Adamantane in Drug Delivery Systems and Surface Recognition. Molecules 2017, 22, 297. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, R.F. Potential for the Repurposing of Adamantane Antivirals for COVID-19. Drugs R D 2021, 21, 267–272. [Google Scholar] [CrossRef]
- Mogensen, S.B.; Taylor, M.K.; Lee, J.-W. Homocoupling Reactions of Azoles and Their Applications in Coordination Chemistry. Molecules 2020, 25, 5950. [Google Scholar] [CrossRef] [PubMed]
- Belousov, Y.A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide azolecarboxylate compounds: Structure, luminescent properties and applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Sathyanarayana, R.; Poojary, B. Exploring recent developments on 1,2,4-triazole: Synthesis and biological applications. J. Chin. Chem. Soc. 2020, 67, 459–477. [Google Scholar] [CrossRef]
- Kovalenko, K.A.; Potapov, A.S.; Fedin, V.P. Micro- and mesoporous metal-organic coordination polymers for separation of hydrocarbons. Russ. Chem. Rev. 2021, 90. [Google Scholar] [CrossRef]
- Lin, R.-B.; Xiang, S.; Zhou, W.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dybtsev, D.N.; Bryliakov, K.P. Asymmetric catalysis using metal-organic frameworks. Coord. Chem. Rev. 2021, 437, 213845. [Google Scholar] [CrossRef]
- Antipin, I.S.; Alfimov, M.V.; Arslanov, V.V.; Burilov, V.A.; Vatsadze, S.Z.; Voloshin, Y.Z.; Volcho, K.P.; Gorbatchuk, V.V.; Gorbunova, Y.G.; Gromov, S.P.; et al. Functional supramolecular systems: Design and applications. Russ. Chem. Rev. 2021, 90, 895–1107. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties. Materials 2020, 13, 2633. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A. Metal ion detection using luminescent-MOFs: Principles, strategies and roadmap. Coord. Chem. Rev. 2020, 415, 213299. [Google Scholar] [CrossRef]
- Rojas, S.; Horcajada, P. Metal–Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chem. Rev. 2020, 120, 8378–8415. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.; Jin, H.; He, D.; Mu, S. Design Engineering, Synthesis Protocols, and Energy Applications of MOF-Derived Electrocatalysts. Nano-Micro Lett. 2021, 13, 132. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Q.; Xie, Q.; Ou, H.; Lin, X.; Zeb, A.; Hu, L.; Wu, Y.; Ma, G. Recent progress in Co–based metal–organic framework derivatives for advanced batteries. J. Mater. Sci. Technol. 2021, 96, 262–284. [Google Scholar] [CrossRef]
- Pettinari, C.; Pettinari, R.; Di Nicola, C.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. [Google Scholar] [CrossRef]
- Xhaferaj, N.; Tăbăcaru, A.; Moroni, M.; Senchyk, G.A.; Domasevitch, K.V.; Pettinari, C.; Galli, S. New Coordination Polymers of Zinc(II), Copper(II) and Cadmium(II) with 1,3-Bis(1,2,4-triazol-4-yl)adamantane. Inorganics 2020, 8, 60. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Lysenko, A.B.; Babaryk, A.A.; Rusanov, E.B.; Krautscheid, H.; Neves, P.; Valente, A.A.; Gonçalves, I.S.; Krämer, K.W.; Liu, S.-X.; et al. Triazolyl–Based Copper–Molybdate Hybrids: From Composition Space Diagram to Magnetism and Catalytic Performance. Inorg. Chem. 2014, 53, 10112–10121. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Lysenko, A.B.; Krautscheid, H.; Rusanov, E.B.; Chernega, A.N.; Krämer, K.W.; Liu, S.X.; Decurtins, S.; Domasevitch, K.V. Functionalized adamantane tectons used in the design of mixed-ligand copper(II) 1,2,4-triazolyl/carboxylate metal-organic frameworks. Inorg. Chem. 2013, 52, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Bukhan’ko, V.O.; Lysenko, A.B.; Krautscheid, H.; Rusanov, E.B.; Chernega, A.N.; Karbowiak, M.; Domasevitch, K.V. AgI/VV Heterobimetallic Frameworks Generated from Novel-Type {Ag2(VO2F2)2(triazole)4} Secondary Building Blocks: A New Aspect in the Design of SVOF Hybrids. Inorg. Chem. 2012, 51, 8025–8033. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Lysenko, A.B.; Boldog, I.; Rusanov, E.B.; Chernega, A.N.; Krautscheid, H.; Domasevitch, K.V. 1,2,4-Triazole functionalized adamantanes: A new library of polydentate tectons for designing structures of coordination polymers. Dalt. Trans. 2012, 41, 8675–8689. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Lysenko, A.B.; Krautscheid, H.; Domasevitch, K.V. “Fluoride molecular scissors”: A rational construction of new Mo(VI) oxofluorido/1,2,4-triazole MOFs. Inorg. Chem. Commun. 2011, 14, 1365–1368. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Lysenko, A.B.; Naumov, D.Y.; Fedin, V.P.; Krautscheid, H.; Domasevitch, K.V. Multiple anion···π interactions with a soft selenium atom: Accommodation of NCSe− anions inside hydrophobic pockets of adamantane/1,2,4-triazole coordination framework. Inorg. Chem. Commun. 2010, 13, 1576–1579. [Google Scholar] [CrossRef]
- Lysenko, A.B.; Senchyk, G.A.; Lincke, J.; Lässig, D.; Fokin, A.A.; Butova, E.D.; Schreiner, P.R.; Krautscheid, H.; Domasevitch, K.V. Metal oxide-organic frameworks (MOOFs), a new series of coordination hybrids constructed from molybdenum(VI) oxide and bitopic 1,2,4-triazole linkers. Dalton Trans. 2010, 39, 4223–4231. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Lysenko, A.B.; Rusanov, E.B.; Chernega, A.N.; Krautscheid, H.; Domasevitch, K.V. Polynuclear and polymeric metal complexes based upon 1,2,4-triazolyl functionalized adamantanes. Inorg. Chim. Acta 2009, 362, 4439–4448. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Lysenko, A.B.; Krautscheid, H.; Sieler, J.; Domasevitch, K.V. A dihydroxidotetracopper(II) framework supported by 4,4’-(adamantane- 1,3-diyl)bis-(1,2,4-triazole) and benzene-1,3,5-tricarboxylate bridges. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2008, 64, 246–249. [Google Scholar] [CrossRef]
- Pavlov, D.I.; Ryadun, A.A.; Samsonenko, D.G.; Fedin, V.P.; Potapov, A.S. Synthesis, crystal structures, and luminescence properties of coordination polymers and a discrete complex of cadmium(II) halides with 1-(1,2,4-triazol-1-yl)adamantane. Russ. Chem. Bull. 2021, 70, 857–863. [Google Scholar] [CrossRef]
- Pavlov, D.I.; Sukhikh, T.S.; Potapov, A.S. Synthesis of azolyl-substituted adamantane derivatives and their coordination compounds. Russ. Chem. Bull. 2020, 69, 1953–1964. [Google Scholar] [CrossRef]
- Pavlov, D.; Sukhikh, T.; Filatov, E.; Potapov, A. Facile Synthesis of 3-(Azol-1-yl)-1-adamantanecarboxylic Acids—New Bifunctional Angle-Shaped Building Blocks for Coordination Polymers. Molecules 2019, 24, 2717. [Google Scholar] [CrossRef] [Green Version]
- Kleywegt, G.J.; Wiesmeijer, W.G.R.; Van Driel, G.J.; Driessen, W.L.; Reedijk, J.; Noordik, J.H. Unidentate versus symmetrically and unsymmetrically bidentate nitrate co-ordination in pyrazole-containing chelates. The crystal and molecular structures of (nitrato-O)[tris (3, 5-dimethylpyrazol-1-ylmethyl) amine] copper (II) nitrate,(nitrato-O, O’)[tris (3, 5-dimethylpyrazol-1-ylmethyl) amine] nickel (II) nitrate, and (nitrato-O)(nitrato-O, O’)[tris (3, 5-dimethylpyrazol-1-ylmethyl) amine] cadmium (II). J. Chem. Soc. Dalt. Trans. 1985, 2177–2184. [Google Scholar] [CrossRef]
- Smirnova, K.S.; Lider, E.V.; Sukhikh, T.S.; Berezin, A.S.; Potapov, A.S. Cadmium coordination compounds with flexible ligand 1,3-bis(1,2,4-triazol-1-yl)propane: Synthesis, structure and luminescent properties. Polyhedron 2020, 177, 114286. [Google Scholar] [CrossRef]
- Marchenko, R.; Potapov, A. 1,3-Bis(1,2,4-triazol-1-yl)adamantane. Molbank 2017, 2017, M968. [Google Scholar] [CrossRef] [Green Version]
- Alemany, P.; Casanova, D.; Alvarez, S.; Dryzun, C.; Avnir, D. Continuous Symmetry Measures: A New Tool in Quantum Chemistry. Rev. Comput. Chem. 2017, 30, 289–352. [Google Scholar]
- Casanova, D.; Alemany, P.; Bofill, J.M.; Alvarez, S. Shape and Symmetry of Heptacoordinate Transition-Metal Complexes: Structural Trends. Chem. A Eur. J. 2003, 9, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Bruker AXS Inc. APEX2 (Version 2.0), SAINT (Version 8.18c), and SADABS (Version 2.11), Bruker Advanced X-Ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2000–2012. [Google Scholar]
- Sheldrick, G.M. IUCr Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]








| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C14H18AgN7O3 | C28H36CdN14O6 | C15.5H24CdN8O7.5 |
| Formula weight | 440.22 | 777.11 | 554.83 |
| Temperature, K | 298(2) | 150(2) | 298(2) |
| Crystal system | monoclinic | monoclinic | monoclinic |
| Space group | P21/c | P21/n | P21/c |
| a, Å | 13.3900(4) | 11.3904(13) | 14.4331(6) |
| b, Å | 9.7545(3) | 13.4621(12) | 12.1592(6) |
| c, Å | 13.0518(4) | 20.904(2) | 25.3803(12) |
| β, ° | 110.4360(10) | 93.070(4) | 104.848(2) |
| Volume, Å3 | 1597.44(8) | 3200.7(6) | 4305.4(3) |
| Z | 4 | 4 | 8 |
| ρcalc, g/cm3 | 1.830 | 1.613 | 1.712 |
| μ, mm−1 | 1.294 | 0.749 | 1.073 |
| F(000) | 888.0 | 1592.0 | 2248.0 |
| Crystal size, mm3 | 0.13 × 0.08 × 0.05 | 0.4 × 0.08 × 0.05 | 0.25 × 0.18 × 0.07 |
| 2Θ range for data collection, ° | 3.246 to 48.81 | 3.6 to 51.39 | 2.92 to 50.052 |
| Index ranges | −15 ≤ h ≤ 15 −11 ≤ k ≤ 11 −15 ≤ l ≤ 15 | −9 ≤ h ≤ 13 −15 ≤ k ≤ 16 −25 ≤ l ≤ 24 | −16 ≤ h ≤ 17 −14 ≤ k ≤ 14 −29 ≤ l ≤ 30 |
| Reflections collected | 16302 | 18657 | 53132 |
| Independent reflections | 2630 [Rint = 0.0390, Rsigma = 0.0298] | 6089 [Rint = 0.0366, Rsigma = 0.0463] | 7600 [Rint = 0.0458, Rsigma = 0.0310] |
| Data/Restraints/Parameters | 2630/50/263 | 6089/0/442 | 7600/33/587 |
| Goodness-of-fit on F2 | 1.047 | 1.007 | 1.168 |
| Final R indexes (I ≥ 2σ (I)) | R1 = 0.0549, wR2 = 0.1346 | R1 = 0.0315, wR2 = 0.0666 | R1 = 0.0724, wR2 = 0.1530 |
| Final R indexes (all data) | R1 = 0.0774, wR2 = 0.1478 | R1 = 0.0465, wR2 = 0.0720 | R1 = 0.0980, wR2 = 0.1631 |
| Largest diff. peak/hole, e·Å−3 | 2.40/−0.97 | 0.75/−0.46 | 1.37/−1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchenko, R.D.; Sukhikh, T.S.; Ryadun, A.A.; Potapov, A.S. Synthesis, Crystal Structure, and Luminescence of Cadmium(II) and Silver(I) Coordination Polymers Based on 1,3-Bis(1,2,4-triazol-1-yl)adamantane. Molecules 2021, 26, 5400. https://doi.org/10.3390/molecules26175400
Marchenko RD, Sukhikh TS, Ryadun AA, Potapov AS. Synthesis, Crystal Structure, and Luminescence of Cadmium(II) and Silver(I) Coordination Polymers Based on 1,3-Bis(1,2,4-triazol-1-yl)adamantane. Molecules. 2021; 26(17):5400. https://doi.org/10.3390/molecules26175400
Chicago/Turabian StyleMarchenko, Roman D., Taisiya S. Sukhikh, Alexey A. Ryadun, and Andrei S. Potapov. 2021. "Synthesis, Crystal Structure, and Luminescence of Cadmium(II) and Silver(I) Coordination Polymers Based on 1,3-Bis(1,2,4-triazol-1-yl)adamantane" Molecules 26, no. 17: 5400. https://doi.org/10.3390/molecules26175400
APA StyleMarchenko, R. D., Sukhikh, T. S., Ryadun, A. A., & Potapov, A. S. (2021). Synthesis, Crystal Structure, and Luminescence of Cadmium(II) and Silver(I) Coordination Polymers Based on 1,3-Bis(1,2,4-triazol-1-yl)adamantane. Molecules, 26(17), 5400. https://doi.org/10.3390/molecules26175400