Volatile Composition and Biological Activity of Jordanian Commercial Samples of R. coriaria L. Fruits
Abstract
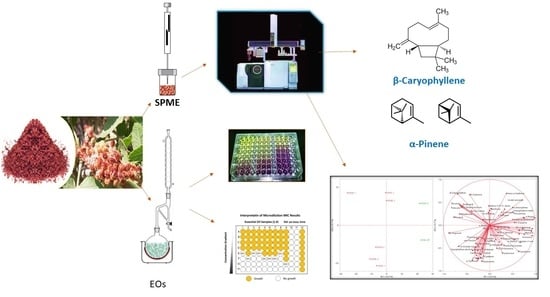
:1. Introduction
2. Results
2.1. The Antioxidant Activity
2.2. Antibacterial Activity
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Essential Oil Extraction
3.3. HS-SPME of Volatile Compounds
3.4. GC-FID and GC-MS Analysis
3.5. GC-MS Analysis of EOs
3.6. Identification of Compounds
3.7. Multivariate Statistical Analysis
3.8. Antioxidant Activity
DPPH (2-Diphenyl-1-picryl-hydrazyl) Free Radical Scavenging Activity
3.9. β-Carotene Bleaching (BCB) Assay
3.10. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- USDA. Germplasm Resources Information Network; Agricultural Research Service: Beltsville, MD, USA, 2007. [Google Scholar]
- Erichsen-Brown, C. Medicinal and Other Uses of North American Plants: A Historical Survey with Special Reference to the Eastern Indian Tribes; Dover Publications: New York, NY, USA, 1989. [Google Scholar]
- Foster, S.; Duke, J.A. A Field Guide to Medicinal Plants: Eastern and Central N. America; Houghton Mifflin Co: Boston, MA, USA, 1990. [Google Scholar]
- Moerman, D. Native American Ethnobotany; Timber Press: Portland, OR, USA, 1998. [Google Scholar]
- Baytop, T. Therapy with Medicinal Plants in Turkey (Past and Present), 1st ed.; Publications of the Istanbul University: Istanbul, Turkey, 1984; No. 3255. (In Turkish) [Google Scholar]
- Dogan, M.; Akgul, A. Characteristics and fatty acid compositions of Rhus coriaria cultivars from Southeast Turkey. Chem. Nat. Compd. 2005, 41, 724–725. [Google Scholar] [CrossRef]
- Gulmez, M.; Oral, N.; Vatansever, L. The effect of water extract of sumac (Rhus coriaria L.) and lactic acid on decontamination and shelf life of raw broiler wings. Poult. Sci. 2006, 85, 1466–1471. [Google Scholar] [CrossRef]
- Uhl, S.R. Handbook of Spices, Seasonings and Flavorings; Technomic Publishing Company, Inc.: Lancaster, PN, USA, 2000. [Google Scholar]
- Rayne, S.; Mazza, G. Biological activities of extracts from sumac (Rhus spp.): A review. Plant Foods Hum. Nutr. 2007, 62, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Bozan, B.; Kosar, M.; Tunalier, Z.; Ozturk, N.; Baser, K. Antioxidant and free radical scavenging activities of Rhus coriaria and Cinnamomum cassia extracts. Acta Aliment. 2003, 32, 53–61. [Google Scholar] [CrossRef]
- Candan, F. Effect of Rhus coriaria L. (Anacardiaceae) on superoxide radical scavenging and xanthine oxidase activity. J. Enzyme Inhib. Med. Chem. 2003, 18, 59–62. [Google Scholar] [CrossRef]
- Candan, F.; Sokmen, A. Effects of Rhus coriaria L. (Anacardiaceae) on lipid peroxidation and free radical scavenging activity. Phytother. Res. 2004, 18, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; Cardile, V.; Santagati, N.A.; Messina, R. Antioxidant and protective effects of sumac leaves on chondrocytes. J. Med. Plants Res. 2009, 3, 855–861. [Google Scholar]
- Pourahmad, J.; Eskandari, M.R.; Shakibaei, R.; Kamalinejad, M. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. against oxidative stress cytotoxicity. Food Chem. Toxicol. 2010, 48, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.V. Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Beretta, G.; Rossoni, G.; Santagati, N.A.; Facino, R.M. Anti-ischemic activity and endothelium-dependent vasorelaxant effect of hydrolysable tannins from the leaves of Rhus coriaria (Sumac) in isolated rabbit heart and thoracic aorta. Planta Med. 2009, 75, 1482–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossah, R.; Nsabimana, C.; Zhang, H.; Chen, W. Optimization of extraction of polyphenols from Syrian sumac (Rhus coriaria L.) and Chinese sumac (Rhus typhina L.) fruits. Res. J. Phytochem. 2010, 4, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Shabbir, A. Rhus coriaria linn, a plant of medicinal, nutritional and industrial importance: A review. J. Anim. Plant Sci. 2012, 22, 505–512. [Google Scholar]
- Abu-Reidah, I.M.; Jamous, R.M.; Ali-Shtayeh, M.S. Phytochemistry, pharmacological properties and industrial applications of Rhus coriaria L. (Sumac). Jordan J. Biol. Sci. 2014, 7, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content: Compositions and gene expression patterns of mono-and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Ebadi, A.; Maggi, F.; Fattahi, R.; Yazdani, D.; Jafari, M. Chemical characterization of the essential oil compositions from Iranian populations of Hypericum perforatum L. Ind. Crops Prod. 2015, 76, 565–573. [Google Scholar] [CrossRef]
- Bahar, B.; Altug, T. Flavour characterization of sumach (Rhus coriaria L.) by means of GC/MS and sensory flavour profile analysis techniques. Int. J. Food Prop. 2009, 12, 379–387. [Google Scholar] [CrossRef]
- Brunke, E.J.; Hammerschmidt, F.J.; Schmaus, G.; Akgül, A. The essential oil of Rhus coriaria L. fruits. Flavour Fragr. J. 1993, 8, 209–214. [Google Scholar] [CrossRef]
- Gharaei, A.; Khajeh, M.; Ghaffari, M.; Choopani, A. Iranian Rhus coriaria (sumac) essential oils extraction. J. Essent. Oil Bear. Plants 2013, 16, 270–273. [Google Scholar] [CrossRef]
- Ghorbani, P.; Namvar, F.; Homayouni-Tabrizi, M.; Soltani, M.; Karimi, E.; Yaghmaei, P. Apoptotic efficacy and antiproliferative potential of silver nanoparticles synthesised from aqueous extract of sumac (Rhus coriaria L.). IET Nanobiotechnol. 2018, 12, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Kurucu, S.; Koyuncu, M.; Güvenç, A.; Baser, K.; Özek, T. The essential oils of Rhus coriaria L. (Sumac). J. Essent. Oil. Res. 1993, 5, 481–486. [Google Scholar] [CrossRef]
- Giovanelli, S.; Giusti, G.; Cioni, P.L.; Minissale, P.; Ciccarelli, D.; Pistelli, L. Aroma profile and essential oil composition of Rhus coriaria fruits from four Sicilian sites of collection. Ind. Crops Prod. 2017, 97, 166–174. [Google Scholar] [CrossRef]
- Elagbar, Z.A.; Shakya, A.K.; Barhoumi, L.M.; Al-Jaber, H.I. Phytochemical Diversity and Pharmacological Properties of Rhus coriaria. Chem. Biodivers. 2020, 17, e1900561. [Google Scholar] [CrossRef]
- Farag, M.A.; Fayek, N.M.; Reidah, I.A. Volatile profiling in Rhus coriaria fruit (sumac) from three different geographical origins and upon roasting as analyzed via solid-phase microextraction. PeerJ 2018, 6, e5121. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Ferk, F.; Simic, T.; Brantner, A.; Dusinska, M.; Kundi, M.; Hoelzl, C.; Nersesyan, A.; Knasmuller, S. DNA-protective effects of sumach (Rhus coriaria L.), a common spice: Results of human and animal studies. Mutat. Res. 2009, 661, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Adwan, G.; Abu-Shanab, B.; Adwan, K. Antibacterial activities of some plant extracts alone and in combination with different antimicrobials against multidrug–resistant Pseudomonas aeruginosa strains. Asian Pac. J. Trop. Med. 2010, 3, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Nimri, L.F.; Meqdam, M.M.; Alkofahi, A. Antibacterial activity of Jordanian medicinal plants. Pharm. Biol. 1999, 37, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, M. Effect of sumach (Rhus coriaria L.) extracts on the oxidative stability of peanut oil. J. Med. Food 2003, 6, 63–66. [Google Scholar] [CrossRef]
- Ozcan, M. Antioxidant activities of rosemary, sage, and sumac extracts and their combinations on stability of natural peanut oil. J. Med. Food 2003, 6, 267–270. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Maggi, F.; Neko, H.T.; Aghdam, M.S. Sumac (Rhus coriaria L.) fruit: Essential oil variability in Iranian populations. Ind. Crop. Prod. 2018, 111, 1–7. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene beta-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Joycharat, N.; Thammavong, S.; Voravuthikunchai, S.P.; Plodpai, P.; Mitsuwan, W.; Limsuwan, S.; Subhadhirasakul, S. Chemical constituents and antimicrobial properties of the essential oil and ethanol extract from the stem of Aglaia odorata Lour. Nat. Prod. Res. 2014, 28, 2169–2172. [Google Scholar] [CrossRef]
- Tanaka, Y.; Uchi, H.; Furue, M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019, 96, 151–158. [Google Scholar] [CrossRef]
- De Carvalho, F.O.; Silva, E.R.; Gomes, I.A.; Santana, H.S.R.; do Nascimento Santos, D.; de Oliveira Souza, G.P.; de Jesus Silva, D.; Monteiro, J.C.M.; de Albuquerque Junior, R.L.C.; de Souza Araujo, A.A.; et al. Anti-inflammatory and antioxidant activity of carvacrol in the respiratory system: A systematic review and meta-analysis. Phytother. Res. 2020, 34, 2214–2229. [Google Scholar] [CrossRef]
- Pombal, S.; Hernandez, Y.; Diez, D.; Mondolis, E.; Mero, A.; Morin-Pinzon, J.; Guerrero, E.I.; Rodilla, J.M. Antioxidant Activity of Carvone and Derivatives against Superoxide Ion. Nat. Prod. Commun. 2017, 12, 653–655. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.C.; Dao, D.Q.; Thong, N.M.; Nam, P.C. Insight into the antioxidant prop-erties of non-phenolic terpenoids contained in essential oils extracted from the buds of Cleistocalyx operculatus: A DFT study. RSC Adv. 2016, 6, 30824–30834. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Elagbar, Z.A.; Naik, R.R.; Shakya, A.K.; Bardaweel, S.K. Fatty Acids Analysis, Antioxidant and Biological Activity of Fixed Oil of Annona muricata L. Seeds. J. Chem. 2016, 2016, 6948098. [Google Scholar] [CrossRef]
- Usman, L.A.; Ismaeel, R.O. Chemical Composition and Antioxidant Potential of Fruit Essential Oil of Laggera pterodonta (DC.) Benth Grown in North Central, Nigeria. J. Biol. Act. Prod. Nat. 2020, 10, 405–410. [Google Scholar]
- Upadhyaya, K.; Dixit, V.K.; Padalia, R.C.; Mathela, C.S. Terpenoid composition and antioxidant activity of essential oil from leaves of Salvia leucantha Cav. J. Essent. Oil Bear. Plants 2009, 12, 551–556. [Google Scholar] [CrossRef]
- Sharma, P.; Shah, G.C. Composition and antioxidant activity of Senecio nudicaulis Wall. ex DC. (Asteraceae): A medicinal plant growing wild in Himachal Pradesh, India. Nat. Prod. Res. 2015, 29, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, D.N.; Costa, J.S.; Oliveira, M.A.; Rabelo, T.K.; Silva, A.; Carvalho, A.A.; Miguel-Dos-Santos, R.; Lauton-Santos, S.; Scotti, L.; Scotti, M.T.; et al. alpha-Terpineol reduces cancer pain via modulation of oxidative stress and inhibition of iNOS. Biomed. Pharmacother. 2018, 105, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Saha, S.; Walia, S.; Ahluwalia, V.; Kaur, C. Antioxidant potential of essential oil and cadinene sesquiterpenes of Eupatorium adenophorum. Toxicol. Environ. Chem. 2013, 95, 127–137. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Mun, S.H.; Kang, O.H.; Joung, D.K.; Kim, S.B.; Choi, J.G.; Shin, D.W.; Kwon, D.Y. In vitro anti-MRSA activity of carvone with gentamicin. Exp. Ther. Med. 2014, 7, 891–896. [Google Scholar] [CrossRef]
- Chen, L.W.; Chung, H.L.; Wang, C.C.; Su, J.H.; Chen, Y.J.; Lee, C.J. Anti-Acne Effects of Cembrene Diterpenoids from the Cultured Soft Coral Sinularia flexibilis. Mar. Drugs 2020, 18, 487. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef] [Green Version]
- Auezova, L.; Najjar, A.; Kfoury, M.; Fourmentin, S.; Greige-Gerges, H. Antibacterial activity of free or encapsulated selected phenylpropanoids against Escherichia coli and Staphylococcus epidermidis. J. Appl. Microbiol. 2020, 128, 710–720. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F.; Viljoen, A.M. The biological activities of 20 Nature Identical Essential Oil Constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Li, L.; Shi, C.; Yin, Z.; Jia, R.; Peng, L.; Kang, S.; Li, Z. Antibacterial activity of alpha-terpineol may induce morphostructural alterations in Escherichia coli. Braz. J. Microbiol. 2014, 45, 1409–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, R.R.; Shakya, A.K.; Khalaf, N.A.; Abuhamdah, S.; Oriquat, G.A.; Maraqa, A.M. GC-MS Analysis and Biological Evaluation of Essential Oil of Zanthoxylum Rhesta (Roxb.) DC Pericarp. Jordan J. Biol. Sci. 2015, 8, 181–193. [Google Scholar]
- Bandeira Reidel, R.V.; Melai, B.; Cioni, P.; Flamini, G.; Pistelli, L. Aroma Profile of Rubus ulmifolius Flowers and Fruits during Different Ontogenetic Phases. Chem. Biodivers. 2016, 13, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectroscopy; Allured: Carol Stream, IL, USA, 2001. [Google Scholar]
- NIST. NIST/EPA/NIH Mass Spectra Library; John Wiley & Sons, Inc.: Hobeken, NJ, USA, 2014. [Google Scholar]
- Al-Hiari, Y.M.; Al-Mazari, I.S.; Shakya, A.K.; Darwish, R.M.; Abu-Dahab, R. Synthesis and antibacterial properties of new 8-nitrofluoroquinolone derivatives. Molecules 2007, 12, 1240–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Antioxidant Activity a | Sample # | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ascorbic Acid | Rutin | EO-1 | EO-2 | EO-3 | EO-4 | EO-5 | EO-6 | EO-7 | EO-8 | |
| DPPH radical scavenging activity | 4.05 | - | 128.5 | 259.1 | 140.1 | 160.9 | 135.8 | 175.4 | 205.3 | 145.5 |
| β-carotene bleaching (BCB) assay | - | 2.93 | 175.2 | 310.5 | 169.5 | 221.5 | 165.5 | 195.0 | 225.0 | 165.1 |
| Microorganism | Sample # | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3.0% DMSO | Ciprofloxacin | EO-1 | EO-2 | EO-3 | EO-4 | EO-5 | EO-6 | EO-7 | EO-8 | |
| Zone of Inhibition (mm) a | ||||||||||
| Staphylococcus aureus ATCC 6538 | - | 24 | 12 | 10 | 13 | 13 | 14 | 13 | 11 | 15 |
| E. coli ATCC 8739 | - | 18 | 9 | 8 | 10 | 9 | 11 | 9 | 8 | 11 |
| Minimum Inhibitory Concentration b (µg/mL) | ||||||||||
| Staphylococcus aureus ATCC 6538 | - | 0.59 | 65.80 | 131.25 | 65.80 | 131.25 | 32.80 | 65.80 | 65.80 | 32.80 |
| E. coli ATCC 8739 | - | 2.34 | 131.25 | 262.50 | 131.25 | 262.50 | 131.25 | 131.25 | 131.25 | 131.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naik, R.R.; Shakya, A.K.; Ferri, B.; Oriquat, G.A.; Pistelli, L.; Numan, N.A.M. Volatile Composition and Biological Activity of Jordanian Commercial Samples of R. coriaria L. Fruits. Molecules 2021, 26, 5691. https://doi.org/10.3390/molecules26185691
Naik RR, Shakya AK, Ferri B, Oriquat GA, Pistelli L, Numan NAM. Volatile Composition and Biological Activity of Jordanian Commercial Samples of R. coriaria L. Fruits. Molecules. 2021; 26(18):5691. https://doi.org/10.3390/molecules26185691
Chicago/Turabian StyleNaik, Rajashri R., Ashok K. Shakya, Benedetta Ferri, Ghaleb A. Oriquat, Luisa Pistelli, and Nawfal A. M. Numan. 2021. "Volatile Composition and Biological Activity of Jordanian Commercial Samples of R. coriaria L. Fruits" Molecules 26, no. 18: 5691. https://doi.org/10.3390/molecules26185691
APA StyleNaik, R. R., Shakya, A. K., Ferri, B., Oriquat, G. A., Pistelli, L., & Numan, N. A. M. (2021). Volatile Composition and Biological Activity of Jordanian Commercial Samples of R. coriaria L. Fruits. Molecules, 26(18), 5691. https://doi.org/10.3390/molecules26185691