Synthesis, Anti-Proliferative Evaluation and Mechanism of 4-Trifluoro Methoxy Proguanil Derivatives with Various Carbon Chain Length
Abstract
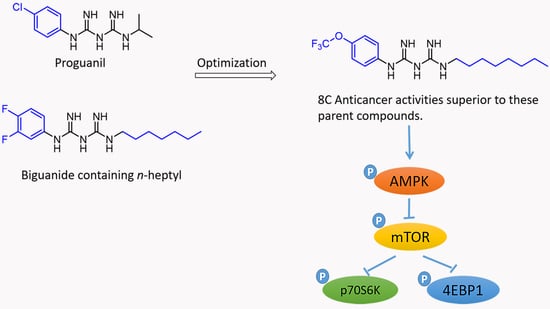
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Method for Synthesizing Compound 2
4.1.2. Method for Synthesizing Compound 2C–10C, 12C
N-1-Ethyl-N-5-(4-trifluoro Methoxy) Proguanil (2C)
N-1-Propyl-N-5-(4-trifluoro Methoxy) Proguanil (3C)
N-1-Butyl-N-5-(4-trifluoro Methoxy) Proguanil (4C)
N-1-Amyl-N-5-(4-trifluoro Methoxy) Proguanil (5C)
N-1-Hexyl-N-5-(4-trifluoro Methoxy) Proguanil (6C)
N-1-Heptyl-N-5-(4-trifluoro Methoxy) Proguanil (7C)
N-1-Octyl-N-5-(4-trifluoro Methoxy) Proguanil (8C)
N-1-Nonyl-N-5-(4-trifluoro Methoxy) Proguanil (9C)
N-1-Decyl-N-5-(4-trifluoro Methoxy) Proguanil (10C)
N-1-Dodecyl-N-5-(4-trifluoro Methoxy) Proguanil (12C)
4.2. Biological Evaluation
4.2.1. Reagents
4.2.2. Cell Lines and Culture Conditions
4.2.3. MTT Cell Viability Assay
4.2.4. Clonogenic Assay
4.2.5. Western Blot Analysis
4.2.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [Green Version]
- JKilgore, J.; Jackson, A.L.; Clark, L.H.; Guo, H.; Zhang, L.; Jones, H.M.; Gilliam, T.P.; Gehrig, P.A.; Zhou, C.; Bae-Jump, V.L. Buformin exhibits anti-proliferative and anti-invasive effects in endometrial cancer cells. Am. J. Transl. Res. 2016, 8, 2705–2715. [Google Scholar]
- Mounkoro, P.; Michel, T.; Meunier, B. Revisiting the mode of action of the antimalarial proguanil using the yeast model. Biochem. Biophys. Res. Commun. 2021, 534, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Gabel, S.A.; Duff, M.R.; Pedersen, L.C.; Derose, E.F.; Krahn, J.M.; Howell, E.E.; London, R.E. A Structural Basis for Biguanide Activity. Biochemistry 2017, 56, 4786–4798. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Feliu, M.; Lord, A.K.; Lukason, D.; Negoro, P.E.; Khan, N.S.; Dagher, Z.; Feldman, M.B.; Reedy, J.L.; Steiger, S.N.; et al. Biguanides enhance antifungal activity against Candida glabrata. Virulence 2018, 9, 1150–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Jiang, W.; Thompson, M.D.; Echeverria, D.; McGinley, J.N.; Thompson, H.J. Effects of Metformin, Buformin, and Phenformin on the Post-Initiation Stage of Chemically Induced Mammary Carcinogenesis in the Rat. Cancer Prev. Res. 2015, 8, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Lea, M.A.; Kim, H.; Desbordes, C. Effects of Biguanides on Growth and Glycolysis of Bladder and Colon Cancer Cells. Anticancer Res. 2018, 38, 5003–5011. [Google Scholar] [CrossRef]
- SBurmaoglu, S.; Algul, O.; Anıl, D.A.; Gobek, A.; Duran, G.G.; Ersan, R.H.; Duran, N. Synthesis and anti-proliferative activity of fluoro-substituted chalcones. Bioorg. Med. Chem. Lett. 2016, 26, 3172–3176. [Google Scholar] [CrossRef] [PubMed]
- Lagu, S.B.; Yejella, R.P.; Bhandare, R.R.; Shaik, A.B. Design, Synthesis, and Antibacterial and Antifungal Activities of Novel Trifluoromethyl and Trifluoromethoxy Substituted Chalcone Derivatives. Pharmaceuticals 2020, 13, 375. [Google Scholar] [CrossRef]
- Leroux, F.R.; Manteau, B.; Vors, J.-P.; Pazenok, S. Trifluoromethyl ethers—Synthesis and properties of an unusual substituent. Beilstein J. Org. Chem. 2008, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Blakemore, D.C.; Narayanan, A.; Unwalla, R.; Lovering, F.; Denny, R.A.; Zhou, H.; Bunnage, M.E. Fluorine in Drug Design: A Case Study with Fluoroanisoles. ChemMedChem 2015, 10, 715–726. [Google Scholar] [CrossRef]
- Mandal, D.; Gupta, R.; Jaiswal, A.K.; Young, R.D. Frustrated Lewis-Pair-Meditated Selective Single Fluoride Substitution in Trifluoromethyl Groups. J. Am. Chem. Soc. 2020, 142, 2572–2578. [Google Scholar] [CrossRef]
- Wang, L.; Wei, J.; Wu, R.; Cheng, G.; Li, X.; Hu, J.; Hu, Y.; Sheng, R. The stability and reactivity of tri-, di-, and monofluoromethyl/methoxy/methylthio groups on arenes under acidic and basic conditions. Org. Chem. Front. 2017, 4, 214–223. [Google Scholar] [CrossRef]
- Deb, U.; Biswas, S. Pretomanid: The latest USFDA-approved anti-tuberculosis drug. Indian J. Tuberc. 2021, 68, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, G.; Pea, F.; Moscarella, E.; Argenziano, G. Sonidegib for the Treatment of Advanced Basal Cell Carcinoma. Front. Oncol. 2020, 10, 582866. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Lu, Z.; Wang, Z.; Zhou, S.; Cao, M.; Deng, J.; Hu, X.; Peng, M.; He, C.; Wu, J.; et al. Synthesis, biological evaluation and anti-proliferative mechanism of fluorine-containing proguanil derivatives. Bioorg. Med. Chem. 2020, 28, 115258. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, D.; Raul, A.D.; Wanjari, P.; Bharatam, P.V. Biguanides: Species with versatile therapeutic applications. Eur. J. Med. Chem. 2021, 219, 113378. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, L.; Cao, M.; Wang, Z.; Xiao, D.; Xu, S.; Deng, J.; Hu, X.; He, C.; Tao, T.; et al. Anticancer properties of novel pyrazole-containing biguanide derivatives with activating the adenosine monophosphate-activated protein kinase signaling pathway. Arch. Pharm. 2019, 352, e1900075. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Song, P.; Hwang, J.S.; Park, H.C.; Kim, K.K.; Son, H.-J.; Kim, Y.-J.; Lee, K.M. Therapeutic Applications of Type 2 Diabetes Mellitus Drug Metformin in Patients with Osteoarthritis. Pharmaceuticals 2021, 14, 152. [Google Scholar] [CrossRef]
- Klein, J.D.; Khanna, I.; Pillarisetti, R.; Hagan, R.A.; LaRocque, L.M.; Rodriguez, E.L.; Sands, J.M. An AMPK activator as a therapeutic option for congenital nephrogenic diabetes insipidus. JCI Insight 2021, 6, e146419. [Google Scholar] [CrossRef]
- Bheemanapally, K.; Ibrahim, M.M.H.; Alshamrani, A.; Briski, K.P. Ventromedial hypothalamic nucleus glycogen regulation of metabolic-sensory neuron AMPK and neurotransmitter expression: Role of lactate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R791–R799. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Muñoz, M.; Contreras, C.; Prieto, D. AMPK, metabolism, and vascular function. FEBS J. 2021, 288, 3746–3771. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Obianom, O.; Huang, J.; Guo, D.; Yang, H.; Li, Q.; Shu, Y. Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation. Pharmaceutics 2021, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.; Ekim, B.; Fingar, D.C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2011, 441, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, C.; Wu, Q.; Tu, Y.; Wang, C.; Yu, X.; Li, B.; Wang, Z.; Sun, S.; Sun, S. MEDAG enhances breast cancer progression and reduces epirubicin sensitivity through the AKT/AMPK/mTOR pathway. Cell Death Dis. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Xie, G.; Sun, L.; Li, Y.; Chen, B.; Wang, C. Periplocin inhibits the growth of pancreatic cancer by inducing apoptosis via AMPK-mTOR signaling. Cancer Med. 2021, 10, 325–336. [Google Scholar] [CrossRef] [PubMed]







| Compound | IC50(μM) ± SD a,b | ||||
|---|---|---|---|---|---|
| J82 | UMUC3 | T24 | OVCAR3 | SKOV3 | |
| Proguanil | 65.6 ± 5.09 | 24.5 ± 2.33 | 32.6 ± 2.91 | 23.1 ± 1.49 | 43.5 ± 3.53 |
| 2C | 5.7 ± 0.21 | 3.7 ± 0.24 | 9.7 ± 0.47 | 4.6 ± 0.45 | 11.2 ± 0.67 |
| 3C | 7.0 ± 0.84 | 1.5 ± 0.17 | 3.2 ± 0.24 | 2.2 ± 0.21 | 20.0 ± 1.74 |
| 4C | 5.0 ± 0.24 | 3.9 ± 0.16 | 4.1 ± 0.29 | 2.0 ± 0.18 | 10.5 ± 0.88 |
| 5C | 2.1 ± 0.18 | 3.9 ± 0.40 | 1.4 ± 0.16 | 1.9 ± 0.22 | 9.1 ± 0.86 |
| 6C | 3.3 ± 0.33 | 2.3 ± 0.21 | 4.3 ± 0.48 | 2.2 ± 0.20 | 7.2 ± 0.62 |
| 7C | 3.3 ± 0.26 | 3.5 ± 0.29 | 3.3 ± 0.23 | 2.1 ± 0.14 | 7.4 ± 0.46 |
| 8C | 2.6 ± 0.23 | 3.3 ± 0.20 | 3.1 ± 0.21 | 3.2 ± 0.34 | 5.5 ± 0.49 |
| 9C | >100 | 32.3 ± 2.54 | 25.5 ± 2.16 | 96.6 ± 7.41 | >100 |
| 10C | >100 | 27.1 ± 1.59 | 21.5 ± 1.51 | 48.9 ± 3.64 | >100 |
| 12C | >100 | 25.1 ± 3.20 | 18.2 ± 1.98 | 43.3 ± 3.52 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Cao, Y.; Luo, Y.; Xiao, D.; Wang, W.; Wang, Z.; Yang, X. Synthesis, Anti-Proliferative Evaluation and Mechanism of 4-Trifluoro Methoxy Proguanil Derivatives with Various Carbon Chain Length. Molecules 2021, 26, 5775. https://doi.org/10.3390/molecules26195775
Xu S, Cao Y, Luo Y, Xiao D, Wang W, Wang Z, Yang X. Synthesis, Anti-Proliferative Evaluation and Mechanism of 4-Trifluoro Methoxy Proguanil Derivatives with Various Carbon Chain Length. Molecules. 2021; 26(19):5775. https://doi.org/10.3390/molecules26195775
Chicago/Turabian StyleXu, Simeng, Yufang Cao, Yu Luo, Di Xiao, Wei Wang, Zhiren Wang, and Xiaoping Yang. 2021. "Synthesis, Anti-Proliferative Evaluation and Mechanism of 4-Trifluoro Methoxy Proguanil Derivatives with Various Carbon Chain Length" Molecules 26, no. 19: 5775. https://doi.org/10.3390/molecules26195775
APA StyleXu, S., Cao, Y., Luo, Y., Xiao, D., Wang, W., Wang, Z., & Yang, X. (2021). Synthesis, Anti-Proliferative Evaluation and Mechanism of 4-Trifluoro Methoxy Proguanil Derivatives with Various Carbon Chain Length. Molecules, 26(19), 5775. https://doi.org/10.3390/molecules26195775