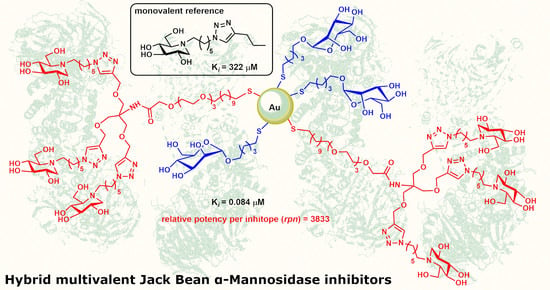
Hybrid Multivalent Jack Bean α-Mannosidase Inhibitors: The First Example of Gold Nanoparticles Decorated with Deoxynojirimycin Inhitopes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Ligands and Gold Nanoparticles
2.2. Biological Evaluation
3. Materials and Methods
3.1. General Experimental Procedures for the Syntheses
3.1.1. Synthesis of Monovalent DNJ Derivative 9
3.1.2. Synthesis of Monovalent DNJ Based Ligand 11
3.1.3. Synthesis of Trivalent DNJ Derivative 16
3.1.4. Synthesis of Trivalent DNJ Based Ligand 17
3.1.5. General Procedure for the In Situ Preparation of Ligands 12 and 18
3.1.6. General Procedure for the Preparation of AuGNPs 1–5
Preparation of 40% monoDNJ-Au-βGlc 1
Preparation of 40% monoDNJ-Au-αMan 2
Preparation of 20% trisDNJ-Au-βGlc 3
Preparation of 20% trisDNJ-Au-αMan 4
Preparation of 40% trisDNJ-Au-αMan 5
3.2. Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Diot, J.; García-Moreno, M.I.; Gouin, S.G.; Ortiz Mellet, C.; Haupt, K.; Kovensky, J. Multivalent iminosugars to modulate affinity and selectivity for glycosidases. Org. Biomol. Chem. 2009, 7, 357–363. [Google Scholar] [CrossRef]
- Compain, P.; Decroocq, C.; Iehl, J.; Holler, M.; Hazelard, D.; Mena Barragán, T.; Ortiz Mellet, C.; Nierengarten, J.-F. Glycosidase Inhibition with Fullerene Iminosugar Balls: A Dramatic Multivalent Effect. Angew. Chem. Int. Ed. 2010, 49, 5753–5756. [Google Scholar] [CrossRef] [PubMed]
- Compain, P.; Bodlenner, A. The multivalent effect in glycosidase inhibition: A new, rapidly emerging topic in glycoscience. ChemBioChem 2014, 15, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Gouin, S.G. Multivalent inhibitors for carbohydrate-processing enzymes: Beyond the “lock-and-key” concept. Chem. Eur. J. 2014, 20, 11616–11628. [Google Scholar] [CrossRef] [PubMed]
- Zelli, R.; Longevial, J.-F.; Dumy, P.; Marra, A. Synthesis and biological properties of multivalent iminosugars. New J. Chem. 2015, 30, 5050–5074. [Google Scholar] [CrossRef]
- Kanfar, N.; Bartolami, E.; Zelli, R.; Marra, A.; Winum, J.-Y.; Ulrich, S.; Dumy, P. Emerging trends in enzyme inhibition by multivalent nanoconstructs. Org. Biomol. Chem. 2015, 13, 9894–9906. [Google Scholar] [CrossRef]
- Matassini, C.; Parmeggiani, C.; Cardona, F.; Goti, A. Are enzymes sensitive to the multivalent effect? Emerging evidence with glycosidases. Tetrahedron Lett. 2016, 57, 5407–5415. [Google Scholar] [CrossRef]
- Compain, P. Multivalent effect in glycosidase inhibition: The end of the beginning. Chem. Rec. 2020, 20, 10–22. [Google Scholar] [CrossRef]
- González-Cuesta, M.; Ortiz Mellet, C.; García Fernández, J.M. Carbohydrate supramolecular chemistry: Beyond the multivalent effect. Chem. Commun. 2020, 56, 5207–5222. [Google Scholar] [CrossRef] [Green Version]
- Lepage, M.L.; Schneider, J.P.; Bodlenner, A.; Meli, A.; De Riccardis, F.; Schmitt, M.; Tarnus, C.; Nguyen-Huynh, N.-T.; Francois, Y.-N.; Leize-Wagner, E.; et al. Iminosugar-Cyclopeptoid Conjugates Raise Multivalent Effect in Glycosidase Inhibition at Unprecedented High Levels. Chem. Eur. J. 2016, 22, 5151–5155. [Google Scholar] [CrossRef]
- Howard, E.; Cousido-Siah, A.; Lepage, M.L.; Schneider, J.P.; Bodlenner, A.; Mitschler, A.; Meli, A.; Izzo, I.; Alvarez, A.; Podjarny, A.; et al. Structural Basis of Outstanding Multivalent Effects in Jack Bean α-Mannosidase Inhibition. Angew. Chem. Int. Ed. 2018, 57, 8002–8006. [Google Scholar] [CrossRef] [Green Version]
- Mirabella, S.; D’Adamio, G.; Matassini, C.; Goti, A.; Delgado, S.; Gimeno, A.; Robina, I.; Moreno-Vargas, A.J.; Šesták, S.; Jiménez-Barbero, J.; et al. Mechanistic Insight into the Binding of Multivalent Pyrrolidines to α-Mannosidases. Chem. Eur. J. 2017, 23, 14585–14596. [Google Scholar] [CrossRef] [PubMed]
- Abellán Flos, M.; García Moreno, M.I.; Ortiz Mellet, C.; García Fernández, J.M.; Nierengarten, J.-F.; Vincent, S.P. Potent Glycosidase Inhibition with Heterovalent Full-erenes: Unveiling the Binding Modes Triggering Multivalent Inhibition. Chem. Eur. J. 2016, 22, 11450–11460. [Google Scholar] [CrossRef] [PubMed]
- Siriwardena, A.; Khanal, M.; Barras, A.; Bande, O.; Mena-Barragán, T.; Ortiz Mellet, C.; García Fernández, J.M.; Boukherroub, R.; Szunerits, S. Unprecedented Inhibition of Glyco-sidase-Catalyzed Substrate Hydrolysis by Nanodiamond-Grafted O-Glycosides. RSC Adv. 2015, 5, 100568–100578. [Google Scholar] [CrossRef] [Green Version]
- Alali, U.; Vallin, A.; Bil, A.; Khanchouche, T.; Mathiron, D.; Przybylski, C.; Beaulieu, R.; Kovensky, J.; Benazza, M.; Bonnet, V. The Uncommon Strong Inhibition of α-Glucosidase by Multivalent Glycoclusters Based on Cyclodextrin Scaffolds. Org. Biomol. Chem. 2019, 17, 7228–7237. [Google Scholar] [CrossRef]
- Marradi, M.; Chiodo, F.; García, I.; Penadés, S. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem. Soc. Rev. 2013, 42, 4728–4745. [Google Scholar] [CrossRef]
- Marradi, M.; García, I.; Penadés, S. Carbohydrate-based nanoparticles for potential applications in medicine. In Nanoparticles in Translational Science and Medicine; Villaverde, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 104, pp. 141–173. ISBN 9780124160200. [Google Scholar]
- Alvarez-Dorta, D.; Brissonnet, Y.; Saumonneau, A.; Deniaud, D.; Bernard, J.; Yan, X.; Tellier, C.; Daligault, F.; Gouin, S.G. Magnetic Nanoparticles Coated with Thiomannosides or Iminosugars to Switch and Recycle Galactosidase Activity. ChemistrySelect 2017, 2, 9552–9556. [Google Scholar] [CrossRef]
- Kleps, I.; Ignat, T.; Miu, M.; Craciunoiu, F.; Trif, M.; Simion, M.; Bragaru, A.; Dinescu, A. Nanostructured Silicon Particles for Medical Applications. J. Nanosci. Nanotechnol. 2010, 10, 2694–2700. [Google Scholar] [CrossRef]
- Bonduelle, C.; Huang, J.; Mena-Barragán, T.; Ortiz Mellet, C.; Decroocq, C.; Etamé, E.; Heise, A.; Compain, P.; Lecommandoux, S. Iminosugar-based glycopolypeptides: Glycosidase inhibition with bioinspired glycoprotein analogue micellar self-assemblies. Chem. Commun. 2014, 50, 3350–3352. [Google Scholar] [CrossRef] [PubMed]
- Matassini, C.; Marradi, M.; Cardona, F.; Parmeggiani, C.; Robina, I.; Moreno-Vargas, A.J.; Penadés, S.; Goti, A. Gold nanoparticles are suitable cores for building tunable iminosugar multivalency. RSC Adv. 2015, 5, 95817–95822. [Google Scholar] [CrossRef] [Green Version]
- Matassini, C.; Vanni, C.; Goti, A.; Morrone, A.; Marradi, M.; Cardona, F. Multimerization of DAB-1 onto Au GNPs affords new potent and selective N-acetylgalactosamine-6-sulfatase (GALNS) inhibitors. Org. Biomol. Chem. 2018, 16, 8604–8612. [Google Scholar] [CrossRef]
- Joosten, A.; Schneider, J.P.; Lepage, M.L.; Tarnus, C.; Bodlenner, A.; Compain, P. A Convergent Strategy for the Synthesis of Second-Generation Iminosugar Clusters Using “Clickable” Trivalent Dendrons. Eur. J. Org. Chem. 2014, 1866–1872. [Google Scholar] [CrossRef]
- Schneider, J.P.; Tommasone, S.; Della Sala, P.; Gaeta, C.; Talotta, C.; Tarnus, C.; Neri, P.; Bodlenner, A.; Compain, P. Synthesis and Glycosidase Inhibition Properties of Ca-lix[8]Arene-Based Iminosugar Click Clusters. Pharmaceuticals 2020, 13, 366–386. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ávila, O.; Hijazi, K.; Marradi, M.; Clavel, C.; Campion, C.; Kelly, C.; Penadés, S. Gold Manno-Glyconanoparticles: Multivalent Systems to Block HIV-1 gp120 Binding to the Lectin DC-SIGN. Chem. Eur. J. 2009, 15, 9874–9888. [Google Scholar] [CrossRef]
- Decroocq, C.; Rodríguez-Lucena, D.; Russo, V.; Mena Barragán, T.; Ortiz Mellet, C.; Compain, P. The Multivalent Effect in Glycosidase Inhibition: Probing the Influence of Architectural Parameters with Cyclodextrin-based Iminosugar Click Clusters. Chem. Eur. J. 2011, 17, 13825–13831. [Google Scholar] [CrossRef]
- Ayesa, S.; Samuelsson, B.; Classon, B. A One-Pot, Solid-Phase Synthesis of Secondary Amines from Reactive Alkyl Halides and an Alkyl Azide. Synlett 2008, 97–99. [Google Scholar]
- Chabre, Y.M.; Contino-Pépin, C.; Placide, V.; Shiao, T.C.; Roy, R. Expeditive synthesis of glycodendrimer scaffolds based on versatile TRIS and mannoside derivatives. J. Org. Chem. 2008, 73, 5602–5605. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.C.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective Ligation of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Manea, F.; Bindoli, C.; Fallarini, S.; Lombardi, G.; Polito, L.; Lay, L.; Bonomi, R.; Mancin, F.; Scrimin, P. Multivalent, Saccharide-Functionalized Gold Nanoparticles as Fully Synthetic Analogs of Type A Neisseria meningitidis Antigens. Adv. Mater. 2008, 20, 4348–4352. [Google Scholar] [CrossRef]
- Chiodo, F.; Enríquez-Navas, P.M.; Angulo, J.; Marradi, M.; Penadés, S. Assembling different antennas of the gp120 high mannose-type glycans on gold nanoparticles provides superior binding to the anti-HIV antibody 2G12 than the individual antennas. Carbohydr. Res. 2015, 405, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.-J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, J.D.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; et al. Alkanethiolate Gold Cluster Molecules with Core Diameters from 1.5 to 5.2 nm: Core and Monolayer Properties as a Function of Core Size. Langmuir 1998, 14, 17–30. [Google Scholar] [CrossRef]
- Zhou, M.; Zeng, C.; Chen, Y.; Zhao, S.; Sfeir, M.Y.; Zhu, M.; Jin, R. Evolution from the plasmon to exciton state in ligand-protected atomically precise gold nanoparticles. Nat. Commun. 2016, 7, 13240. [Google Scholar] [CrossRef]
- Decroocq, C.; Joosten, A.; Sergent, R.; Mena Barragan, T.; Ortiz Mellet, C.; Compain, P. The Multivalent Effect in Glycosidase Inhibition: Probingthe Influence of Valency, Peripheral Ligand Structure, and Topology with Cyclodextrin-Based Iminosugar ClickClusters. ChemBioChem 2013, 14, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Segel, I.H. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems; John Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]






| AuGNP | DNJ Conc. (μM) in 2 mg mL−1 AuGNP 1 | Ki (μM) 2 | rpn3 |
|---|---|---|---|
| 1 | 413 | 16 ± 2 | 20 |
| 2 | 467 | 8 ± 2 | 40 |
| 3 | 567 | 0.198 ± 0.060 | 1626 |
| 4 | 450 | 0.175 ± 0.171 | 1840 |
| 5 | 503 | 0.084 ± 0.066 | 3833 |
| 6 | 0 | n.i. 4 | _ |
| 7 | 0 | n.i. 4 | _ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanni, C.; Bodlenner, A.; Marradi, M.; Schneider, J.P.; Ramirez, M.d.l.A.; Moya, S.; Goti, A.; Cardona, F.; Compain, P.; Matassini, C. Hybrid Multivalent Jack Bean α-Mannosidase Inhibitors: The First Example of Gold Nanoparticles Decorated with Deoxynojirimycin Inhitopes. Molecules 2021, 26, 5864. https://doi.org/10.3390/molecules26195864
Vanni C, Bodlenner A, Marradi M, Schneider JP, Ramirez MdlA, Moya S, Goti A, Cardona F, Compain P, Matassini C. Hybrid Multivalent Jack Bean α-Mannosidase Inhibitors: The First Example of Gold Nanoparticles Decorated with Deoxynojirimycin Inhitopes. Molecules. 2021; 26(19):5864. https://doi.org/10.3390/molecules26195864
Chicago/Turabian StyleVanni, Costanza, Anne Bodlenner, Marco Marradi, Jérémy P. Schneider, Maria de los Angeles Ramirez, Sergio Moya, Andrea Goti, Francesca Cardona, Philippe Compain, and Camilla Matassini. 2021. "Hybrid Multivalent Jack Bean α-Mannosidase Inhibitors: The First Example of Gold Nanoparticles Decorated with Deoxynojirimycin Inhitopes" Molecules 26, no. 19: 5864. https://doi.org/10.3390/molecules26195864
APA StyleVanni, C., Bodlenner, A., Marradi, M., Schneider, J. P., Ramirez, M. d. l. A., Moya, S., Goti, A., Cardona, F., Compain, P., & Matassini, C. (2021). Hybrid Multivalent Jack Bean α-Mannosidase Inhibitors: The First Example of Gold Nanoparticles Decorated with Deoxynojirimycin Inhitopes. Molecules, 26(19), 5864. https://doi.org/10.3390/molecules26195864