Prebiotic Impacts of Soybean Residue (Okara) on Eubiosis/Dysbiosis Condition of the Gut and the Possible Effects on Liver and Kidney Functions
Abstract
:1. Introduction
Dietary Fiber in Nutrition
2. Nutritional and Anti-Nutritional Components of Soy and Soy Residue
2.1. Nutritional Constituents
2.2. Anti-Nutritional/Bioactive Constituents of Soybean Co-Products; with Emphasis on Polyphenols (Soy Isoflavones)
3. Valorization of Soybean Residue (Okara) as a Functional Food
3.1. Application of Soybean Residue in Human Nutrition
3.2. Application of Soybean Residue in Animal Nutrition
4. Physicochemical and Prebiotic Influence of Dietary Fiber (Emphasis on Okara-Derived Fiber) on Gut and Associated Tissues: Gut, Liver, and Kidneys
4.1. Physicochemical Role of Dietary Fiber in The Gastrointestinal Tract
4.2. The Use of Soybean Residue as a Prebiotic
4.3. Prebiotic Status of Soybean Residue on Gut Microbiome
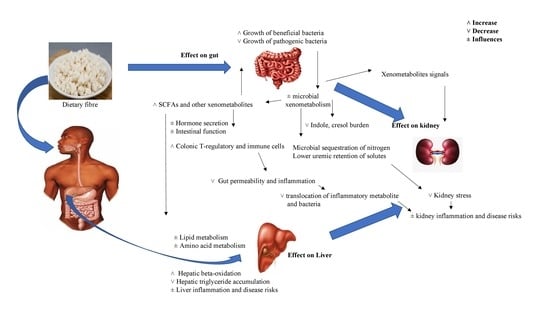
4.4. Gut, Liver, and Kidney Responses to the Prebiotic Effects of Dietary Fiber in General
4.4.1. Gut Responses
4.4.2. Liver Responses
4.4.3. Kidney Responses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LDL | Low-density lipoprotein |
| VLDL | Very-low-density lipoprotein |
| FDA | Food and Drug Administration |
| SDF | Soluble dietary fiber |
| IDF | Insoluble dietary fiber |
| CDK | Chronic kidney disease |
| NAFLD | Non-alcoholic fatty liver disease |
| SCFAs | Short-chain fatty acids |
| Hif-1α | Hypoxia-inducible factor-1 alpha |
| ZO-1 | Zonula occludens-1 |
| LPS | Lipopolysaccharides |
| GLP-2 | Glucagon-like peptide-2 |
| NASH | Non-alcoholic steatohepatitis |
| MtS | Metabolic syndrome |
| FAS | Fatty acid synthesis |
| Olfr-78 | Olfactory receptor 78 |
| HHP | High hydrostatic pressure |
| ACE | Angiotensin-converting enzyme |
References
- Colletti, A.; Attrovio, A.; Boffa, L.; Mantegna, S.; Cravotto, G. Valorisation of By-Products from Soybean (Glycine max (L.) Merr.) Processing. Molecules 2020, 25, 2129. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Kerwin, S. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr. Med. Chem. Anti-Cancer Agents 2004, 4, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.W.; Kim, S.Y.; Jee, S.H.; Kim, Y.N.; Nam, C.M. Soy food consumption and risk of prostate cancer: A meta-analysis of observational studies. Nutr. Cancer 2009, 61, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Watanabe, S.; Setchell, K.D. Report on the 8th international symposium on the role of soy in health promotion and chronic disease prevention and treatment. J. Nutr. 2009, 139, 796S–802S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva-Suárez, M.-J.; Pérez-Cózar, M.-L.; Mateos-Aparicio, I.; Redondo-Cuenca, A. Potential fat-lowering and prebiotic effects of enzymatically treated okara in high-cholesterol-fed Wistar rats. Int. J. Food Sci. Nutr. 2016, 67, 828–833. [Google Scholar] [CrossRef]
- O’Toole, D.K. Characteristics and use of okara, the soybean residue from soy milk production a review. J. Agric. Food Chem. 1999, 47, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Jimeénez-Escrig, A.; Tenorio, M.D.; Espinosa-Martos, I.; Rupeérez, P. Health-promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J. Agric. Food Chem. 2008, 56, 7495–7501. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Liu, Y.; Li, B. Okara dietary fiber and hypoglycemic effect of okara foods. Bioact. Carbohydr. Diet. Fibre 2013, 2, 126–132. [Google Scholar] [CrossRef]
- Zhong-Hua, L.; Hong-Lian, G.; Rui-Ling, L.; Jin-Hui, Z. Extraction and Antioxidant Activity of Soybean Saponins from Lowtemperature Soybean Meal by MTEH. Open Biotechnol. J. 2015, 9, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, M.; Yokoyama, W.; Hong, Y.; Barttley, G.; Rupérez, P. Effect of high-fat diets supplemented with okara soybean by-product on lipid profiles of plasma, liver and faeces in Syrian hamsters. Food Chem. 2011, 124, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.; Charchoghlyan, H.; Lee, J.S.; Kim, M. Bioactive compounds and antioxidant activities of the Korean lotus leaf (Nelumbo nucifera) condiment: Volatile and nonvolatile metabolite profiling during fermentation. Int. J. Food Sci. Technol. 2015, 50, 1988–1995. [Google Scholar] [CrossRef]
- Hu, Y.; Piao, C.; Chen, Y.; Zhou, Y.; Wang, D.; Yu, H.; Xu, B. Soybean residue (okara) fermentation with the yeast Kluyveromyces marxianus. Food Biosci. 2019, 31, 100439. [Google Scholar] [CrossRef]
- Kang, M.J.; Bae, I.Y.; Lee, H.G. Rice noodle enriched with okara: Cooking property, texture, and in vitro starch digestibility. Food Biosci. 2018, 22, 178–183. [Google Scholar] [CrossRef]
- Nagai, T.; Te Li, L.; Ma, Y.L.; Sarkar, P.K.; Nout, R.; Park, K.Y.; Jeong, J.K.; Lee, J.E.; Im Lee, G.; Lee, C.H. Diversity of plant-based food products involving alkaline fermentation. In Handbook Indigenous Foods Involving Alkaline Fermentation; CRC Press: Boca Raton, FL, USA, 2014; pp. 7–187. [Google Scholar]
- Chan, L.Y.; Takahashi, M.; Lim, P.J.; Aoyama, S.; Makino, S.; Ferdinandus, F.; Ng, S.Y.C.; Arai, S.; Fujita, H.; Tan, H.C. Eurotium cristatum fermented okara as a potential food ingredient to combat diabetes. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rastall, R.A.; Gibson, G.R. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr. Opin. Biotechnol. 2015, 32, 42–46. [Google Scholar] [CrossRef]
- Abdallah, A.; Zhang, P.; Zhong, Q.; Sun, Z. Application of traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug Metab. 2019, 20, 54–64. [Google Scholar] [CrossRef]
- Kieffer, D.A.; Martin, R.J.; Adams, S.H. Impact of dietary fibers on nutrient management and detoxification organs: Gut, liver, and kidneys. Adv. Nutr. 2016, 7, 1111–1121. [Google Scholar] [CrossRef] [Green Version]
- Parnell, J.A.; Raman, M.; Rioux, K.P.; Reimer, R.A. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012, 32, 701–711. [Google Scholar] [CrossRef]
- Rossi, M.; Johnson, D.W.; Campbell, K.L. The kidney–Gut axis: Implications for nutrition care. J. Ren. Nutr. 2015, 25, 399–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.; De Maria, S.; Cartenì, M.; Nardone, G. Gut–liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Che, D.; Hailong, J.; Zhao, B.; Rui, H.; Danquah, K.; Qin, G. Effects of pulverized oyster mushroom (Pleurotus ostreatus) on diarrhea incidence, growth performance, immunity, and microbial composition in piglets. J. Sci. Food Agric. 2019, 99, 3616–3627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, M.A.; Monro, J.A.; Brennan, C.S. Effect of inclusion of soluble and insoluble fibres into extruded breakfast cereal products made with reverse screw configuration. Int. J. Food Sci. Technol. 2008, 43, 2278–2288. [Google Scholar] [CrossRef]
- Symons, L.; Brennan, C. The effect of barley β-glucan fiber fractions on starch gelatinization and pasting characteristics. J. Food Sci. 2004, 69, FCT257–FCT261. [Google Scholar] [CrossRef]
- Brennan, C.S. Dietary fibre, glycaemic response, and diabetes. Mol. Nutr. Food Res. 2005, 49, 560–570. [Google Scholar] [CrossRef]
- Lunn, J.; Buttriss, J. Carbohydrates and dietary fibre. Nutr. Bull. 2007, 32, 21–64. [Google Scholar] [CrossRef]
- Chhabra, S. Dietary Fibre-Nutrition and Health Benefits. In Functional Food and Human Health; Springer: Singapore, 2018; pp. 15–25. [Google Scholar]
- Li, Q.; Yang, S.; Li, Y.; Huang, Y.; Zhang, J. Antioxidant activity of free and hydrolyzed phenolic compounds in soluble and insoluble dietary fibres derived from hulless barley. LWT 2019, 111, 534–540. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Chawla, R.; Patil, G. Soluble dietary fiber. Compr. Rev. Food Sci. Food Saf. 2010, 9, 178–196. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J. In vitro hypoglycemic and cholesterol lowering effects of dietary fiber prepared from cocoa (Theobroma cacao L.) shells. Food Funct. 2012, 3, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Schroeder, N.M. Dietary treatments for childhood constipation: Efficacy of dietary fiber and whole grains. Nutr. Rev. 2013, 71, 98–109. [Google Scholar] [CrossRef]
- De Moraes Crizel, T.; Jablonski, A.; de Oliveira Rios, A.; Rech, R.; Flôres, S.H. Dietary fiber from orange byproducts as a potential fat replacer. LWT-Food Sci. Technol. 2013, 53, 9–14. [Google Scholar] [CrossRef]
- Bikker, P.; Dirkzwager, A.; Fledderus, J.; Trevisi, P.; Le Huërou-Luron, I.; Lalleès, J.P.; Awati, A. The effect of dietary protein and fermentable carbohydrates levels on growth performance and intestinal characteristics in newly weaned piglets. J. Anim. Sci. 2006, 84, 3337–3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.; Che, D.; Qin, G.; Rui, H.; Sello, C.T.; Hailong, J. Interactions of dietary fibre with nutritional components on gut microbial composition, function and health in monogastrics. Curr. Protein Pept. Sci. 2018, 19, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Kong, X.; Che, D.; Qin, G.; Jiang, H. Effects of dietary supplementation of alfalfa (Medicago sativa) fibre on the blood biochemistry, nitrogen metabolism, and intestinal morphometry in weaning piglets. Appl. Ecol. Environ. Res. 2019, 17, 2275–2295. [Google Scholar] [CrossRef]
- Adams, S.; Sello, C.T.; Qin, G.-X.; Che, D.; Han, R. Does dietary fiber affect the levels of nutritional components after feed formulation? Fibers 2018, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, A.; Costabile, A.; Martin-Pelaez, S.; Vitaglione, P.; Klinder, A.; Gibson, G.R.; Fogliano, V. Potential prebiotic activity of oligosaccharides obtained by enzymatic conversion of durum wheat insoluble dietary fibre into soluble dietary fibre. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 283–290. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, S.; Lule, V.K.; Malik, R.K.; Tomar, S.K. Soy Bioactive components in functional perspective: A review. Int. J. Food Prop. 2016, 19, 2550–2574. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Isoflavone supplements for menopausal women: A systematic review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vong, W.C.; Liu, S.-Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Kamble, D.B.; Rani, S. Bioactive components, in vitro digestibility, microstructure and application of soybean residue (okara): A review. Legume Sci. 2020, 2, e32. [Google Scholar] [CrossRef] [Green Version]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suárez, M. Isolation and characterisation of cell wall polysaccharides from legume by-products: Okara (soymilk residue), pea pod and broad bean pod. Food Chem. 2010, 122, 339–345. [Google Scholar] [CrossRef]
- Li, B.; Qiao, M.; Lu, F. Composition, nutrition, and utilization of okara (soybean residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; de Oliveira Filho, J.G.; de Santana Silva, J.; de Sousa, M.F.; da Silva Vilela, M.; da Silva, M.A.P.; Lemes, A.C.; Egea, M.B. Okara flour: Its physicochemical, microscopical and functional properties. Nutr. Food Sci. 2019. [Google Scholar] [CrossRef]
- Pérez-López, E.; Veses, A.; Redondo, N.; Tenorio-Sanz, M.; Villanueva, M.; Redondo-Cuenca, A.; Marcos, A.; Nova, E.; Mateos-Aparicio, I.; Rupérez, P. Soybean Okara modulates gut microbiota in rats fed a high-fat diet. Bioact. Carbohydr. Diet. Fibre 2018, 16, 100–107. [Google Scholar] [CrossRef]
- Guimarães, R.M.; Ida, E.I.; Falcão, H.G.; de Rezende, T.A.M.; de Santana Silva, J.; Alves, C.C.F.; da Silva, M.A.P.; Egea, M.B. Evaluating technological quality of okara flours obtained by different drying processes. LWT 2020, 123, 109062. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Jiménez-Escrig, A.; Rupérez, P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydr. Polym. 2010, 82, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Cuenca, A.; Villanueva-Suárez, M.J.; Mateos-Aparicio, I. Soybean seeds and its by-product okara as sources of dietary fibre. Measurement by AOAC and Englyst methods. Food Chem. 2008, 108, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Rupérez, P. High hydrostatic pressure improves the functionality of dietary fibre in okara by-product from soybean. Innov. Food Sci. Emerg. Technol. 2010, 11, 445–450. [Google Scholar] [CrossRef]
- Muliterno, M.M.; Rodrigues, D.; de Lima, F.S.; Ida, E.I.; Kurozawa, L.E. Conversion/degradation of isoflavones and color alterations during the drying of okara. LWT 2017, 75, 512–519. [Google Scholar] [CrossRef]
- Chan, W.M.; Ma, C.Y. Modification of proteins from soymilk residue (okara) by trypsin. J. Food Sci. 1999, 64, 781–786. [Google Scholar] [CrossRef]
- Singh, A.; Meena, M.; Kumar, D.; Dubey, A.K.; Hassan, M.I. Structural and functional analysis of various globulin proteins from soy seed. Crit. Rev. Food Sci. Nutr. 2015, 55, 1491–1502. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Liu, W.-S.; Kwok, K.C.; Kwok, F. Isolation and characterization of proteins from soymilk residue (okara). Food Res. Int. 1996, 29, 799–805. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Alaiz, M.; Vioque, J.; Rupérez, P. Health-promoting activities of ultra-filtered okara protein hydrolysates released by in vitro gastrointestinal digestion: Identification of active peptide from soybean lipoxygenase. Eur. Food Res. Technol. 2010, 230, 655–663. [Google Scholar] [CrossRef]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Jankovic, V.S.; Vucelic-Radovic, B.V. Bioactive proteins and energy value of okara as a byproduct in hydrothermal processing of soy milk. J. Agric. Food Chem. 2013, 61, 9210–9219. [Google Scholar] [CrossRef]
- Yu, B.; Lu, Z.-X.; Bie, X.-M.; Lu, F.-X.; Huang, X.-Q. Scavenging and anti-fatigue activity of fermented defatted soybean peptides. Eur. Food Res. Technol. 2008, 226, 415–421. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K.; Muhammed, A.; Jeyaram, K.; Talukdar, N.C. Enhancement of antioxidant properties of two soybean varieties of Sikkim Himalayan region by proteolytic Bacillus subtilis fermentation. J. Funct. Foods 2015, 14, 650–658. [Google Scholar] [CrossRef]
- Li, F.-J.; Yin, L.-J.; Cheng, Y.-Q.; Yamaki, K.; Fan, J.-F.; Li, L.-T. Comparison of angiotensin I-converting enzyme inhibitor activities of pre-fermented Douchi (a Chinese traditional fermented soybean food) started with various cultures. Int. J. Food Eng. 2009, 5. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides-opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.Y.; Jang, J.S.; Hong, S.M.; Lee, J.E.; Sung, S.R.; Park, H.R.; Park, S. Long-term consumption of fermented soybean-derived Chungkookjang enhances insulinotropic action unlike soybeans in 90% pancreatectomized diabetic rats. Eur. J. Nutr. 2007, 46, 44–52. [Google Scholar] [CrossRef]
- Wang, W.; Bringe, N.A.; Berhow, M.A.; Gonzalez de Mejia, E. β-Conglycinins among sources of bioactives in hydrolysates of different soybean varieties that inhibit leukemia cells in vitro. J. Agric. Food Chem. 2008, 56, 4012–4020. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Tatsumi, E.; Ding, C.-H.; Li, L.-T. Angiotensin I-converting enzyme inhibitory peptides in douchi, a Chinese traditional fermented soybean product. Food Chem. 2006, 98, 551–557. [Google Scholar] [CrossRef]
- Cho, S.-J.; Oh, S.-H.; Pridmore, R.D.; Juillerat, M.A.; Lee, C.-H. Purification and characterization of proteases from Bacillus amyloliquefaciens isolated from traditional soybean fermentation starter. J. Agric. Food Chem. 2003, 51, 7664–7670. [Google Scholar] [CrossRef]
- Varghese, T.; Pare, A. Effect of microwave assisted extraction on yield and protein characteristics of soymilk. J. Food Eng. 2019, 262, 92–99. [Google Scholar] [CrossRef]
- De Toledo, T.; Canniatti-Brazaca, S.; Arthur, V.; Piedade, S. Effects of gamma radiation on total phenolics, trypsin and tannin inhibitors in soybean grains. Radiat. Phys. Chem. 2007, 76, 1653–1656. [Google Scholar] [CrossRef]
- Yalcin, S.; Basman, A. Effects of infrared treatment on urease, trypsin inhibitor and lipoxygenase activities of soybean samples. Food Chem. 2015, 169, 203–210. [Google Scholar] [CrossRef]
- Tao, X.; Cai, Y.; Liu, T.; Long, Z.; Huang, L.; Deng, X.; Zhao, Q.; Zhao, M. Effects of pretreatments on the structure and functional properties of okara protein. Food Hydrocoll. 2019, 90, 394–402. [Google Scholar] [CrossRef]
- Pereira, D.G.; Justus, A.; Falcão, H.G.; Rocha, T. d. S.; Ida, E.I.; Kurozawa, L.E. Enzymatic hydrolysis of okara protein concentrate by mixture of endo and exopeptidase. J. Food Process. Preserv. 2019, 43, e14134. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, L.; Tao, X.; Su, J.; Chen, B.; Zhao, M.; Zhao, Q.; Van der Meeren, P. Adjustment of the structural and functional properties of okara protein by acid precipitation. Food Biosci. 2020, 37, 100677. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suárez, M.-J.; Zapata-Revilla, M.-A.; Tenorio-Sanz, M.-D. Pea pod, broad bean pod and okara, potential sources of functional compounds. LWT-Food Sci. Technol. 2010, 43, 1467–1470. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, S.K.-C. Selected odor compounds in soymilk as affected by chemical composition and lipoxygenases in five soybean materials. J. Agric. Food Chem. 2007, 55, 426–431. [Google Scholar] [CrossRef]
- Quitain, A.T.; Oro, K.; Katoh, S.; Moriyoshi, T. Recovery of oil components of okara by ethanol-modified supercritical carbon dioxide extraction. Bioresour. Technol. 2006, 97, 1509–1514. [Google Scholar] [CrossRef]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Zilic, S.M.; Kresovic, M.M.; Vucelic-Radovic, B.V. Mineral elements, lipoxygenase activity, and antioxidant capacity of okara as a byproduct in hydrothermal processing of soy milk. J. Agric. Food Chem. 2014, 62, 9017–9023. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, W.; Xu, B. Food quality improvement of soy milk made from short-time germinated soybeans. Foods 2013, 2, 198–212. [Google Scholar] [CrossRef] [Green Version]
- Swallah, M.S.; Fu, H.; Sun, H.; Affoh, R.; Yu, H. The Impact of Polyphenol on General Nutrient Metabolism in the Monogastric Gastrointestinal Tract. J. Food Qual. 2020, 2020, 5952834. [Google Scholar] [CrossRef]
- Singh, B.; Yadav, D.; Vij, S. Soybean bioactive molecules: Current trend and future prospective. In Bioactive Molecules in Food; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 1–29. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean bioactive peptides and their functional properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhang, Z.; Huang, H.; Li, Z. Health benefits of soy and soy phytochemicals. AME Med. J. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Gambacorta, G.; La Notte, E. Evaluation of isoflavone content and antioxidant activity of soy-wheat pasta. Int. J. Food Sci. Technol. 2009, 44, 1304–1313. [Google Scholar] [CrossRef]
- Villares, A.; Rostagno, M.A.; García-Lafuente, A.; Guillamón, E.; Martínez, J.A. Content and profile of isoflavones in soy-based foods as a function of the production process. Food Bioprocess Technol. 2011, 4, 27–38. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Nef, S. Soy, phytoestrogens and metabolism: A review. Mol. Cell. Endocrinol. 2009, 304, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhu, D.; Li, K.; Yang, Y.; Lei, Z.; Zhang, Z. Soybean curd residue: Composition, utilization, and related limiting factors. ISRN Ind. Eng. 2013, 2013, 423590. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Preedy, V.R. Isoflavones: Chemistry, analysis, function and effects. In Royal Society Chemistry; RSC Publishing: Cambridge, UK, 2012. [Google Scholar]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Jackson, C.-J.; Dini, J.; Lavandier, C.; Rupasinghe, H.; Faulkner, H.; Poysa, V.; Buzzell, D.; DeGrandis, S. Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochem. 2002, 37, 1117–1123. [Google Scholar] [CrossRef]
- Balisteiro, D.M.; Rombaldi, C.V.; Genovese, M.I. Protein, isoflavones, trypsin inhibitory and in vitro antioxidant capacities: Comparison among conventionally and organically grown soybeans. Food Res. Int. 2013, 51, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, Y.; Mishra, S.; Bisaria, V. Microbial β-glucosidases: Cloning, properties, and applications. Crit. Rev. Biotechnol. 2002, 22, 375–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Yang, L.; Xu, J.Z.; Yeung, S.Y.V.; Huang, Y.; Chen, Z.-Y. Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem. 2005, 90, 735–741. [Google Scholar] [CrossRef]
- Voss, G.; Rodríguez-Alcalá, L.; Valente, L.; Pintado, M. Impact of different thermal treatments and storage conditions on the stability of soybean byproduct (okara). J. Food Meas. Charact. 2018, 12, 1981–1996. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Galanakis, C.; Goulas, V.; Tsakona, S.; Manganaris, G.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Chebil, L.; Humeau, C.; Anthoni, J.; Dehez, F.; Engasser, J.-M.; Ghoul, M. Solubility of flavonoids in organic solvents. J. Chem. Eng. Data 2007, 52, 1552–1556. [Google Scholar] [CrossRef]
- Genovese, M.I.; Lajolo, F.M. Isoflavones in soy-based foods consumed in Brazil: Levels, distribution, and estimated intake. J. Agric. Food Chem. 2002, 50, 5987–5993. [Google Scholar] [CrossRef]
- Vong, W.C.; Lim, X.Y.; Liu, S.-Q. Biotransformation with cellulase, hemicellulase and Yarrowia lipolytica boosts health benefits of okara. Appl. Microbiol. Biotechnol. 2017, 101, 7129–7140. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.-C.; Liu, Y.-M.; Shiau, S.-Y. Effect of okara and vital gluten on physico-chemical properties of noodle. Czech J. Food Sci. 2018, 36, 301–306. [Google Scholar]
- Kamble, D.B.; Singh, R.; Rani, S.; Pratap, D. Physicochemical properties, in vitro digestibility and structural attributes of okara-enriched functional pasta. J. Food Process. Preserv. 2019, 43, e14232. [Google Scholar] [CrossRef]
- Wu, S. Preparation of bean dregs cake. Food Ind. 2003, 24, 23–24. [Google Scholar]
- Zhao, G.-L.; Kong, J. Enzymolysis bean dregs biscuit development. Food Res. Dev. 2009, 10, 67–69. [Google Scholar]
- Park, J.; Choi, I.; Kim, Y. Cookies formulated from fresh okara using starch, soy flour and hydroxypropyl methylcellulose have high quality and nutritional value. LWT-Food Sci. Technol. 2015, 63, 660–666. [Google Scholar] [CrossRef]
- Suda, T.; Kido, Y.; Tsutsui, S.; Tsutsui, D.; Fujita, M.; Nakaya, Y. Nutritional evaluation of the new OKARA powder for food processing material. Foods Food Ingred. J. Jpn. 2007, 212, 320. [Google Scholar]
- Yao, X.; Song, W.; Zhang, Y.; Xiao, W. On the application of enzyme on preparations in bread containing soybeand. Cereal Feed Ind. 2006, 11, 22–23. [Google Scholar]
- Wang, Y.; Tang, J. Application of soybean fiber on bread. J. Zhengzhou Inst. Technol. 2000, 21, 75–77. [Google Scholar]
- Sun, X.; Yang, Y. Study on cooking quality of noodle of okara fiber. Grain Process 2010, 1, 57–59. [Google Scholar]
- Katayama, M.; Wilson, L.A. Utilization of okara, a byproduct from soymilk production, through the development of soy-based snack food. J. Food Sci. 2008, 73, S152–S157. [Google Scholar] [CrossRef] [PubMed]
- Bedani, R.; Campos, M.M.; Castro, I.A.; Rossi, E.A.; Saad, S.M. Incorporation of soybean by-product okara and inulin in a probiotic soy yoghurt: Texture profile and sensory acceptance. J. Sci. Food Agric. 2014, 94, 119–125. [Google Scholar] [CrossRef]
- Yang, C.-M. Soybean milk residue ensiled with peanut hulls: Fermentation acids, cell wall composition, and silage utilization by mixed ruminal microorganisms. Bioresour. Technol. 2005, 96, 1419–1424. [Google Scholar] [CrossRef]
- Wong, M.H.; Tang, L.; Kwok, F. The use of enzyme-digested soybean residue for feeding common carp. Biomed. Environ. Sci. 2003, 9, 418–423. [Google Scholar]
- Yang, C.; Gu, J. Study on the active okara for feeding egg chicken. Feed Ind. 1997, 18, 26–27. [Google Scholar]
- Wang, Z.; Jiang, W.; Hu, Z.; Wang, L. Feeding effects on finishing cattle by substituting soybean meal by dry bean curd pulp. J. Yellow Cattle Sci. 2004, 30, 15–17. [Google Scholar]
- Hermann, J.; Honeyman, M.S. Okara: A possible high protein feedstuff for organic pig diets. Anim. Ind. Rep. 2004, 650, 601–604. [Google Scholar]
- Wang, Z.-H.; Wang, L.-Z.; Chen, Y.-S.; Wu, Z.-F. Contrast trial of substituting dried tofu pulp for soybean meal in dairy diet. China Dairy Cattle 2003, 2, 24–26. [Google Scholar]
- Junyi, Q.; Fuyuan, Z. Research on the processing of microbial protein feed by mixed culture solid-state fermentation on soybean waste. Feed Ind. 2008, 22, 21–24. [Google Scholar]
- Pan, T.; Zhang, D.; Zhao, C.; Li, K. Study on microbial protein production by mixed culture solid state fermentation on soybean waste. Chem. Bioeng. 2004, 6, 35–41. [Google Scholar]
- Nagano, T.; Hirano, R.; Kurihara, S.; Nishinari, K. Improved effects of okara atomized by a water jet system on α-amylase inhibition and butyrate production by Roseburia intestinalis. Biosci. Biotechnol. Biochem. 2020, 84, 1467–1474. [Google Scholar] [CrossRef]
- Pérez-López, E.; Cela, D.; Costabile, A.; Mateos-Aparicio, I.; Rupérez, P. In vitro fermentability and prebiotic potential of soyabean Okara by human faecal microbiota. Br. J. Nutr. 2016, 116, 1116–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Préstamo, G.; Rupérez, P.; Espinosa-Martos, I.; Villanueva, M.J.; Lasunción, M.A. The effects of okara on rat growth, cecal fermentation, and serum lipids. Eur. Food Res. Technol. 2007, 225, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Corpuz, H.M.; Arimura, M.; Chawalitpong, S.; Miyazaki, K.; Sawaguchi, M.; Nakamura, S.; Katayama, S. Oral Administration of Okara Soybean By-Product Attenuates Cognitive Impairment in a Mouse Model of Accelerated Aging. Nutrients 2019, 11, 2939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-S.; Yu, O.-K.; Byun, M.-S.; Cha, Y.-S. Okara, a soybean by-product, prevents high fat diet-induced obesity and improves serum lipid profiles in C57BL/6J mice. Food Sci. Biotechnol. 2016, 25, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Katsukawa, M.; Tanaka, H.; Fukuda, H.; Okuno, S.; Tsuda, K.; Iritani, N. Okara ameliorates glucose tolerance in GK rats. J. Clin. Biochem. Nutr. 2016, 58, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Nguyen, T.H.; Nguyen, L.T.; Kamoshita, S.; Tran, T.P.; Le, H.T.; Shimura, F.; Yamamoto, S. Okara Improved Blood Glucose Level in Vietnamese with Type 2 Diabetes Mellitus. J. Nutr. Sci. Vitaminol. 2019, 65, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, R.M.; Silva, T.E.; Lemes, A.C.; Boldrin, M.C.F.; da Silva, M.A.P.; Silva, F.G.; Egea, M.B. Okara: A soybean by-product as an alternative to enrich vegetable paste. LWT 2018, 92, 593–599. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, J.; Zhang, Z.; Zhang, W.; Yang, J.; Liu, L.; Yang, Z.; Zeng, X. Insights into the structural characteristics and in vitro starch digestibility on steamed rice bread as affected by the addition of okara. Food Hydrocoll. 2020, 113, 106533. [Google Scholar] [CrossRef]
- Bedani, R.; Vieira, A.D.S.; Rossi, E.A.; Saad, S.M.I. Tropical fruit pulps decreased probiotic survival to in vitro gastrointestinal stress in synbiotic soy yoghurt with okara during storage. LWT-Food Sci. Technol. 2014, 55, 436–443. [Google Scholar] [CrossRef]
- Adams, S.; Xiangjie, K.; Hailong, J.; Guixin, Q.; Sossah, F.L.; Dongsheng, C. Prebiotic effects of alfalfa (Medicago sativa) fiber on cecal bacterial composition, short-chain fatty acids, and diarrhea incidence in weaning piglets. RSC Adv. 2019, 9, 13586–13599. [Google Scholar] [CrossRef] [Green Version]
- Satchithanandam, S.; Vargofcak-Apker, M.; Calvert, R.J.; Leeds, A.R.; Cassidy, M.M. Alteration of gastrointestinal mucin by fiber feeding in rats. J. Nutr. 1990, 120, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Piel, C.; Montagne, L.; Sève, B.; Lalleès, J.-P. Increasing digesta viscosity using carboxymethylcellulose in weaned piglets stimulates ileal goblet cell numbers and maturation. J. Nutr. 2005, 135, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, J.T. Mucus: The front line of intestinal mucosal defense. Ann. N. Y. Acad. Sci. 1992, 664, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Billings, G.; Sigal, M.; Lichtman, J.S.; Hansson, G.C.; Elias, J.E.; Amieva, M.R.; Huang, K.C.; Sonnenburg, J.L. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 2015, 18, 478–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Slaughter, S.L.; Ellis, P.R.; Jackson, E.C.; Butterworth, P.J. The effect of guar galactomannan and water availability during hydrothermal processing on the hydrolysis of starch catalysed by pancreatic α-amylase. Biochim. Iophysica Acta Gen. Subj. 2002, 1571, 55–63. [Google Scholar] [CrossRef]
- Dona, A.C.; Pages, G.; Gilbert, R.G.; Kuchel, P.W. Digestion of starch: In vivo and in vitro kinetic models used to characterise oligosaccharide or glucose release. Carbohydr. Polym. 2010, 80, 599–617. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef] [Green Version]
- Gray, J. Dietary Fibre: Definition, Analysis, Physiology Health; International Life Sciences Institute (ILSI): Brussels, Belgium, 2006; p. 36. [Google Scholar]
- Sasaki, T.; Kohyama, K. Influence of non-starch polysaccharides on the in vitro digestibility and viscosity of starch suspensions. Food Chem. 2012, 133, 1420–1426. [Google Scholar] [CrossRef]
- Nagano, T.; Arai, Y.; Yano, H.; Aoki, T.; Kurihara, S.; Hirano, R.; Nishinari, K. Improved physicochemical and functional properties of okara, a soybean residue, by nanocellulose technologies for food development–A review. Food Hydrocoll. 2020, 109, 105964. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.-M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT-Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bliss, D.Z.; Stein, T.P.; Schleifer, C.R.; Settle, R.G. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am. J. Clin. Nutr. 1996, 63, 392–398. [Google Scholar] [CrossRef]
- Johnston, K.; Thomas, E.L.; Bell, J.D.; Frost, G.; Robertson, M.D. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet. Med. 2009, 27, 391–397. [Google Scholar] [CrossRef]
- Espinosa-Martos, I.; Rupérez, P. Indigestible fraction of okara from soybean: Composition, physicochemical properties and in vitro fermentability by pure cultures of Lactobacillus acidophilus and Bifidobacterium bifidum. Eur. Food Res. Technol. 2009, 228, 685–693. [Google Scholar] [CrossRef]
- Bedani, R.; Rossi, E.A.; Saad, S.M.I. Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiol. 2013, 34, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Villanueva-Suárez, M.J.; Pérez-Cózar, M.L.; Redondo-Cuenca, A. Sequential extraction of polysaccharides from enzymatically hydrolyzed okara byproduct: Physicochemical properties and in vitro fermentability. Food Chem. 2013, 141, 1114–1119. [Google Scholar] [CrossRef]
- Tu, Z.; Chen, L.; Wang, H.; Ruan, C.; Zhang, L.; Kou, Y. Effect of fermentation and dynamic high pressure microfluidization on dietary fibre of soybean residue. J. Food Sci. Technol. 2014, 51, 3285–3292. [Google Scholar] [CrossRef] [Green Version]
- Kitawaki, R.; Takagi, N.; Iwasaki, M.; Asao, H.; Okada, S.; Fukuda, M. Plasma cholesterol-lowering effects of soymilk and okara treated by lactic acid fermentation in rats. J. Jpn. Soc. Food Sci. Technol. 2007, 54, 379–382. [Google Scholar] [CrossRef]
- Kitawaki, R.; Nishimura, Y.; Takagi, N.; Iwasaki, M.; Tsuzuki, K.; Fukuda, M. Effects of Lactobacillus fermented soymilk and soy yogurt on hepatic lipid accumulation in rats fed a cholesterol-free diet. Biosci. Biotechnol. Biochem. 2009, 73, 1484–1488. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. Embo Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alisi, A.; Ceccarelli, S.; Panera, N.; Nobili, V. Causative role of gut microbiota in non-alcoholic fatty liver disease pathogenesis. Front. Cell. Infect. Microbiol. 2012, 2, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brun, P.; Castagliuolo, I.; Leo, V.D.; Buda, A.; Pinzani, M.; Palù, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Hijová, E.; Bertková, I.; Štofilová, J. Dietary fibre as prebiotics in nutrition. Cent. Eur. J. Public Health 2019, 27, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Livingston, K.A.; Sawicki, C.M.; McKeown, N.M.; Obin, M.; Roberts, S.B.; Chung, M. Dietary Fiber and the Human Gut Microbiota: Application of Evidence Mapping Methodology. Nutrients 2017, 9, 125. [Google Scholar]
- Hijova, E.; Chmelarova, A. Short chain fatty acids and colonic health. Bratisl. Lek. Listy 2007, 108, 354. [Google Scholar] [PubMed]
- Goodlad, R.; Ratcliffe, B.; Fordham, J.; Wright, N. Does dietary fibre stimulate intestinal epithelial cell proliferation in germ free rats? Gut 1989, 30, 820–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; O’Riordan, M.X. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Tambuwala, M.M.; Cummins, E.P.; Lenihan, C.R.; Kiss, J.; Stauch, M.; Scholz, C.C.; Fraisl, P.; Lasitschka, F.; Mollenhauer, M.; Saunders, S.P. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 2010, 139, 2093–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karhausen, J.; Furuta, G.T.; Tomaszewski, J.E.; Johnson, R.S.; Colgan, S.P.; Haase, V.H. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Investig. 2004, 114, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Martin, R.J.; Raggio, A.M.; McCutcheon, K.L.; Brown, I.L.; Birkett, A.; Newman, S.S.; Skaf, J.; Hegsted, M.; Tulley, R.T. High-amylose resistant starch increases hormones and improves structure and function of the gastrointestinal tract: A microarray study. Lifestyle Genom. 2012, 5, 26–44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chen, S.; Duan, F.; Liu, A.; Li, S.; Zhong, W.; Sheng, W.; Chen, J.; Xu, J.; Xiao, S. Prebiotics enhance the biotransformation and bioavailability of ginsenosides in rats by modulating gut microbiota. J. Ginseng. Res. 2020. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, T.; Sasaki, M.; Araki, Y.; Okamoto, T.; Yasuoka, T.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T. Effects of the soluble fibre pectin on intestinal cell proliferation, fecal short chain fatty acid production and microbial population. Digestion 2003, 67, 42–49. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Ohata, A.; Usami, M.; Miyoshi, M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 2005, 21, 838–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mithieux, G.; Andreelli, F.; Magnan, C. Intestinal gluconeogenesis: Key signal of central control of energy and glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 419–423. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Masciana, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Lecerf, J.-M.; Dépeint, F.; Clerc, E.; Dugenet, Y.; Niamba, C.N.; Rhazi, L.; Cayzeele, A.; Abdelnour, G.; Jaruga, A.; Younes, H. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 2012, 108, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease two-hit process: Multifactorial character of the second hit. Hippokratia 2009, 13, 128. [Google Scholar]
- Cortez-Pinto, H.; Jesus, L.; Barros, H.; Lopes, C.; Moura, M.; Camilo, M. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin. Nutr. 2006, 25, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Galisteo, M.; Duarte, J.; Zarzuelo, A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008, 19, 71–84. [Google Scholar] [CrossRef]
- Federico, A.; Zulli, C.; de Sio, I.; Del Prete, A.; Dallio, M.; Masarone, M.; Loguercio, C. Focus on emerging drugs for the treatment of patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 16841. [Google Scholar] [CrossRef]
- Duarte, S.M.B.; Stefano, J.T.; Vanni, D.S.; Carrilho, F.J.; Oliveira, C.P.M.S.D. Impact of current diet at the risk of non-alcoholic fatty liver disease (NAFLD). Arq. Gastroenterol. 2019, 56, 431–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.M. Effects of inulin on lipid parameters in humans. J. Nutr. 1999, 129, 1471S–1473S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, N.; Roberfroid, M.; Delzenne, N. Dietary oligofructose modifies the impact of fructose on hepatic triacylglycerol metabolism. Metab. Clin. Exp. 1996, 45, 1547–1550. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: A dose–response study in JCR: LA-cp rats. Br. J. Nutr. 2010, 103, 1577–1584. [Google Scholar] [CrossRef] [Green Version]
- Delzenne, N.M.; Williams, C.M. Prebiotics and lipid metabolism. Curr. Opin. Lipidol. 2002, 13, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Levrat, M.-A.; Favier, M.-L.; Moundras, C.; Rémésy, C.; Demigné, C.; Morand, C. Role of dietary propionic acid and bile acid excretion in the hypocholesterolemic effects of oligosaccharides in rats. J. Nutr. 1994, 124, 531–538. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Mafra, D.; Fouque, D. Gut Microbiota and Inflammation in Chronic Kidney Disease Patients. Clin. Kidney J. 2015, 8, 332–334. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Jiang, H.; Shi, K.; Ren, Y.; Zhang, P.; Cheng, S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology 2012, 17, 733–738. [Google Scholar] [CrossRef]
- Wu, I.-W.; Hsu, K.-H.; Lee, C.-C.; Sun, C.-Y.; Hsu, H.-J.; Tsai, C.-J.; Tzen, C.-Y.; Wang, Y.-C.; Lin, C.-Y.; Wu, M.-S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 938–947. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Johnson, D.; Xu, H.; Carrero, J.; Pascoe, E.; French, C.; Campbell, K. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Younes, H.; Alphonse, J.-C.; Behr, S.R.; Demigné, C.; Rémésy, C. Role of fermentable carbohydrate supplements with a low-protein diet in the course of chronic renal failure: Experimental bases. Am. J. Kidney Dis. 1999, 33, 633–646. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Shoaie, S.; Bergentall, M.; Ghaffari, P.; Zhang, C.; Larsson, E.; Bäckhed, F.; Nielsen, J. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol. Syst. Biol. 2015, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Bergen, W.G. Small-intestinal or colonic microbiota as a potential amino acid source in animals. Amino Acids 2015, 47, 251–258. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.-X.; Rey, F.; Wang, T. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [Green Version]
- Remuzzi, G.; Perico, N.; Macia, M.; Ruggenenti, P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int. 2005, 68, S57–S65. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Soy Products | Treatments (Bacterial Fermentation) | Health Benefits | References |
|---|---|---|---|
| Starter culture | |||
| Fermented soybean | Bacillus subtilis SHZ, B. subtilis MTCC 5480 | Antioxidant | [62,63] |
| Douchi qu | B. subtilis natto, B. subtilis B1 | ACE inhibitory | [64] |
| Cheonggukjang | B. licheniformis SCD 111067P | Antihypertensive, Antidiabetic | [65,66] |
| Soy Products | Treatments (Fungi Fermentation) | Health Benefits | References |
| Starter culture | |||
| Douchi qu | Aspergillus oryzae, Mucor wutungkiao | ACE inhibitory | [64] |
| Douchi | Aspergillus oryzae, Aspergillus egyptiacus | Antioxidant, Antihypertensive | [67,68] |
| Meju | Aspergillus oryzae | Antimicrobial | [69] |
| Soy Products | Heat Treatments | Effects | References |
| Soymilk | Microwave-assisted extraction | Increase protein content, viscosity, protein solubility, and digestibility | [70] |
| Raw soybean | Gamma irradiation | Increase total phenolic content, Decrease tannins and trypsin inhibitors | [71] |
| Soybeans | Infrared treatment | Inactivated both trypsin inhibitors and lipoxygenase | [72] |
| Micro Components | Amount (mg/100 g Dry Matter) |
|---|---|
| Thiamine (B1) | 0.48–0.59 |
| Niacin (B3) | 0.82–1.04 |
| Riboflavin (B2) | 0.03–0.04 |
| Mn | 0.2–3.1 |
| Zn | 0.3–3.5 |
| Cu | 0.1–1.2 |
| Fe | 0.6–11 |
| Na | 16–96 |
| Ca | 260–428 |
| K | 936–1350 |
| Mg | 130–165 |
| Macro Components | Amount (g/100 g Dry Matter) |
| Ash | 3.0–4.5 |
| Dietary fiber | 42.4–58.1 |
| Soluble dietary fiber | 4.2–14.6 |
| Insoluble dietary fiber | 40.2–50.8 |
| Fat | 8.3–10.9 |
| Carbohydrate | 3.8–5.3 |
| Protein | 15.2–33.4 |
| Phytochemicals | Amount (g/100 g Dry Matter) |
| Isoflavone glucosides | 10.3 |
| Isoflavone aglycones | 5.41 |
| Malonyl glucosides | 19.7 |
| Acetyl glucosides | 0.32 |
| Saponins | 0.1 |
| Phytic acid | 0.5–1.2 |
| Experimental Model | Effect | Conclusion Remarks | Reference |
|---|---|---|---|
| In vivo (Wistar rats fed high-fat diet), fed okara (20%), for four weeks. | ↓ body weight, ↓ triglycerides, ↑ SCFA production, ↑ amino acid metabolism, ↑ mineral absorption, ↑ microbial protection; ↔ Firmicutes: Bacteroidetes ratio, Bacteroides and B. coccoides-E. rectale groups in control group, ↑ C. leptum and Bacteroides population in feces, Enterobacteriaceae (cecal content) and Enterococcus (fecal and cecal content) groups. | Okara exerts health-promoting attributes in vivo and could be further used as prebiotic and functional ingredient in foods | [51] |
| In vivo (Female Wistar rats fed a standard rat diet) fed dietary rich okara (10%), for four weeks | ↓ body weight gain, ↓ total cholesterol, ↑ antioxidant status and butyrogenic effect in the cecum, ↑ apparent absorption and true retention of calcium | The development of an innovative soybean by-product rich in dietary fiber could be useful as a functional ingredient with health-promoting benefits | [8] |
| In vivo (High-cholesterol-fed Wistar rats), for four weeks | ↓ liver and serum triglyceride levels, ↓ pH of fecal contents, ↑ total lipids, triglycerides and bile acids in feces, ↑ SCFA production. | Enzymatically treated okara fiber can improve intestinal transit by increasing fecal bulk. | [6] |
| In vitro (Water jet (WJ) treated okara and water jet treated microcrystalline cellulose (MCC)), effect on α-amylase inhibition and butyrate production using Roseburia intestinalis | ↑ inhibition of α-amylase activities by WJ-treated okara than WJ-treated MCC, ↑ butyrate production by Roseburia intestinalis in WJ-treated okara | These results depict that WJ system can be used on okara to improve inhibited α-amylase activities and butyrate production by gut microbiota. | [125] |
| In vivo (High fat-fed Syrian hamsters), fed 13% or 20% okara fiber for 3 weeks | ↑ fecal excretion of total lipids, triglycerides, free cholesterol, and total nitrogen. ↔ feed intake and body weight gain. 20% okara group. ↓ plasma triglycerides, VLDL- plus LDL cholesterol and total cholesterol, ↓ liver total lipids, triglycerides, and total esterified cholesterol concentrations. | Okara might aid in the prevention of hyperlipidemia and could be used as a natural ingredient for functional food preparation. | [12] |
| In vitro (Fermentability and prebiotic potential of okara using human fecal slurries), using 16S rRNA-based fluorescence in situ hybridization, and HPLC | ↑ SCFA plus lactic acid, ↑ beneficial bacteria (bifidobacterial and lactobacilli), ↓ potentially harmful bacterial groups (clostridia and Bacteroides) | The differences observed between fructo-oligosaccharides and okara substrates could be accredited to the great complexity of okara’s cell wall, which requires longer times to be fermented than other easily digested molecules. Hence, allowing an extended potential prebiotic effect. These findings support an in vitro potential prebiotic effect of Okara. | [126] |
| In vivo (Wistar Hannover female rats), control group (fed standard rat chow) and treated group (fed a mixture of the standard rat chow plus okara), for 4 weeks | ↔ food intake, ↓ growth rate, and feeding efficiency, ↑ fecal weight and moisture, ↓ lower pH, ↑ cecal weight, ↑ total SCFA production in okara-fed group than control group. ↔ albumin, uric acid, protein, bilirubin, or glucose content in rat serum for both groups. | Okara is a rich source of low-cost dietary fiber and protein, and might be effective as a dietary weight-loss supplement with a potential prebiotic effect. | [127] |
| In vivo (Senescence-accelerated mouse prone 8 (SAMP8) mice), fed standard diet, or a diet containing (7.5% or 15%, w/w) okara, for 26 weeks | 15% okara-fed group; ↓ body weight, ↑ fecal weight, and altered cecal microbiota composition compared with the control group, ↔ serum lactic acid, and butyric acid levels. 7.5% okara-fed group; ↑ NeuN intensity in the hippocampus than control mice, ↓ inflammatory cytokine TNF-α, ↑ brain-derived neurotrophic factor, ↑acetylcholine synthesizing enzyme ↑ acetylcholine level in the brain | Oral administration of okara could delay cognitive decline without drastically changing gut microbiota | [128] |
| In vivo (high fat-fed C57BL/6J male mice), for 12 weeks | ↓ body weight and epididymal fat weight. ↓ serum and hepatic lipid profiles. ↑ fecal triacylglycerol and total cholesterol levels. ↑ PPAR-α expression, ↓ PPAR-γ and FAS levels | Okara consumption appears to protect mice against diet-induced obesity and metabolic dysregulation related to obesity | [129] |
| In vivo (high lard fed Goto-Kakizaki (GK) Type 2 diabetes Male rats), for 2 weeks | ↔ body weight gain or food intake, ↓ plasma glucose levels, ↑ mRNA expression levels of PPARγ, adiponectin, and GLUT4, | The study suggested that okara can play a significant role in treating type 2 diabetes. | [130] |
| In vivo (Human Type 2 Diabetes Mellitus outpatients), fed okara for 2 weeks | ↑ food intake (fiber 6.9 to 12.6 g), ↓ Fasting blood glucose (6.3 to 5.4 mmol/L), ↓ fructosamine (319 to 301 μmol/L) | Okara increased fiber intake and consequently improved blood glucose in DM patients | [131] |
| Experimental Model | Dietary Formulations | Effect on Food Properties and Function | Description | Reference |
|---|---|---|---|---|
| In vitro (Okara and vital gluten on physicochemical properties of noodle). | Added portion of okara (0%, 5% & 10%) | ↑ total phenolics, flavonoids and radical-scavenging activity. 10–15% okara ↓ optimum cooking time, extensibility tensile strength, and elasticity of noodle. | 5% or 10% dried okara powder plus 6% vital gluten might be best in producing noodles with increased phytochemicals and consumer’s sensory acceptability. | [106] |
| In vitro (Application of okara to enrich vegetable paste) | High moisture (80.77–81.42%). Low lipid (5.62–7.62%). Low calorie (95.14–108.14 kcal). | ↑ β-carotene (0.411 mg/100 mL). ↑ antioxidant activity. ↑ isoflavones (0.15 μmol/gFM). | The sample with the lowest content of okara (34 g/100 g) presented the highest average of 8.0 in the acceptance test and was also considered the tasters’ favorite one. | [132] |
| In vitro (Starch digestibility of steamed rice bread fortify with okara) | Added portion of okara (0%, 7%, 14% & 21%) | ↑ Elasticity and viscidity. ↓ Hardness, cohesiveness, and chewiness. ↑ Amylose content, slowly digestible starch, resistant starch. ↓ Predicted glycemic index (pGI) from 79.14 to 74.17–68.91 | Okara can potentially modify the texture and starch digestibility of steamed rice bread. | [133] |
| In vitro (Gastrointestinal stress in synbiotic soy yogurt with okara during storage), for 28 days | Soy yogurt + Okara, Soy yogurt + Guava pulp Soy yogurt + Mango pulp | ↑ Survival rates (%) of L. acidophilus La-5 and B. animalis Bb-12, ranging from 8 to 9 log cfu/g after simulated gastrointestinal conditions. ↑ Probiotic strains functionality. | In this study, okara endorsed probiotic functionality in simulated gastrointestinal conditions, however, the addition of fruit pulps might lead to a reduction. | [134] |
| In vitro (Digestibility of rice noodle enriched with okara) | Added portion of okara (0%, 5%, 10%, & 20%). | ↑ cooking loss, adhesiveness, and hardness with increasing level of okara. ↓ water absorption, cohesiveness, and swelling index. 0%, 5% & 10% okara ↓ in vitro starch digestibility. 10% okara ↓ predicted glycemic index | 10% okara can be used to produce health-beneficial rice noodles with reduced in vitro starch digestibility and improve cooking quality. | . [15] |
| In vitro (Digestibility and structural attributes of okara-enriched functional pasta) | Added okara contents (10–50%) | ↔ structural changes, ↓ glycemic index (27.41 ± 0.05–12.38 ± 0.01). 50% okara ↑ total phenolic content and antioxidant activity (158.37 ± 0.40 to 232.90 ± 0.85 mg GAE/100 g and 10.87 ± 0.10%–56.21 ± 0.05%) | The study indicated that pasta enriched with okara has the potential to be commercialized on the industrial level to develop nutritional enriched functional pasta. | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swallah, M.S.; Fan, H.; Wang, S.; Yu, H.; Piao, C. Prebiotic Impacts of Soybean Residue (Okara) on Eubiosis/Dysbiosis Condition of the Gut and the Possible Effects on Liver and Kidney Functions. Molecules 2021, 26, 326. https://doi.org/10.3390/molecules26020326
Swallah MS, Fan H, Wang S, Yu H, Piao C. Prebiotic Impacts of Soybean Residue (Okara) on Eubiosis/Dysbiosis Condition of the Gut and the Possible Effects on Liver and Kidney Functions. Molecules. 2021; 26(2):326. https://doi.org/10.3390/molecules26020326
Chicago/Turabian StyleSwallah, Mohammed Sharif, Hongliang Fan, Sainan Wang, Hansong Yu, and Chunhong Piao. 2021. "Prebiotic Impacts of Soybean Residue (Okara) on Eubiosis/Dysbiosis Condition of the Gut and the Possible Effects on Liver and Kidney Functions" Molecules 26, no. 2: 326. https://doi.org/10.3390/molecules26020326
APA StyleSwallah, M. S., Fan, H., Wang, S., Yu, H., & Piao, C. (2021). Prebiotic Impacts of Soybean Residue (Okara) on Eubiosis/Dysbiosis Condition of the Gut and the Possible Effects on Liver and Kidney Functions. Molecules, 26(2), 326. https://doi.org/10.3390/molecules26020326