Chemosensitization of HT29 and HT29-5FU Cell Lines by a Combination of a Multi-Tyrosine Kinase Inhibitor and 5FU Downregulates ABCC1 and Inhibits PIK3CA in Light of Their Importance in Saudi Colorectal Cancer
Abstract
:1. Introduction
2. Results
2.1. Criteria of the Saudi CRC Patients
2.2. Tyrosine and Serine/Threonine Activities in the CRC Samples
2.2.1. Enrichment Pathway Analysis
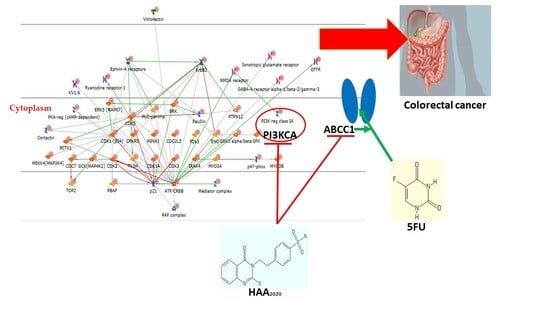
2.2.2. Network and GO Processes
2.3. Real-Time PCR of the CRC Pateints’ Samples
2.4. Combination Cytotoxicity and Selectivity Studies
2.5. Real-Time PCR of ABC Transporters in HT29, HCT116, HT-5FU and HCT116-5FU Cells
2.6. Real-Time PCR and Western Blotting in HT29 and HT29-5FU Cells
2.7. Cell Cycle Perturbation of HT29 and HT29-5FU Cells
2.8. Detection of Apoptosis in HT29 and HT29-5FU Cells
2.9. Immunofluorescence Microscopy and Western Blotting in HT29 and HT29-5FU Cells
3. Discussion
4. Materials and Methods
4.1. Ethical Approval, Selection of the Saudi CRC Patients, and Sampling
4.2. 5FU, LY294002, and HAA2020
4.3. Maintenance of Cell Lines
4.4. Kinase Cctivity in the CRC Samples
Data Interpretation
4.5. Quantitative Real-Time PCR
4.6. Combination Cytotoxicity and Selectivity Studies
4.7. Western Blotting
4.8. Cell Cycle Perturbation
4.9. Determination of Apoptosis
4.10. Immunofluorescence Microscopy
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ABC | ATP binding cassette transporters |
| AKT/1 | V-akt murine thymoma viral oncogene/homolog 1 |
| AKT2 | V-akt murine thymoma viral oncogene homolog 2 |
| APC | Adenomatosis polyposis coli |
| ATP5A1 | ATP synthase subunit alpha |
| CTCFL | CCCTC-binding factor (zinc finger protein)-like |
| ERK | extracellular signal regulated kinase |
| FITC | fluorescein isothiocyanate |
| FLT-3 | Fms like tyrosine kinase 3 |
| HER2 | Erb-B2 receptor tyrosine kinase 2 |
| JAK | Janus kinase |
| KFSHRC | King Fisal Specialist Hospital and Research Center |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| KIT | KIT Proto-Oncogene |
| MAPK | mitogen activated protein kinase |
| mTOR | mechanistic target of rapamycin |
| NARS | Asparaginyl-TRNA synthetase |
| NOS | nitric oxide synthase |
| PARP-1 | Poly(ADP-Ribose) polymerase 1 |
| PDGFRA | platelet-derived growth factor receptor alpha |
| PDPK1 | 3-phosphoinositide dependent protein kinase 1 |
| PI | propidium iodide |
| PI3K | phosphoinositide-3-kinase |
| PIK3CA | phosphatidylinositol-4,5-bisphosphate 3-kinase regulatory subunit alpha |
| PIK3CB | phosphatidylinositol-4,5-bisphosphate 3-kinase regulatory subunit beta |
| PIK3CD | phosphatidylinositol-4,5-bisphosphate 3-kinase regulatory subunit delta |
| PIK3CG | phosphatidylinositol-4,5-bisphosphate 3-kinase regulatory subunit gamma |
| PIK3R1 | phosphatidylinositol-4,5-bisphosphate 3-kinase regulatory subunit 1 |
| PIK3R3 | phosphatidylinositol-4,5-bisphosphate 3-kinase regulatory subunit 2 |
| PKC | protein kinase C |
| PLC | phospholipase C |
| PLCG1 | phospholipase C gamma 1 |
| RAF | Raf-1 proto-oncogene |
| RAS | Kirsten rat sarcoma oncogene 2-delete |
| ROS | ROS proto-oncogene 1 |
| SMAD4 | Mothers against DPP homolog 4 (drosophila) |
| Src | proto-oncogene tyrosine-protein kinase |
| STAT | signal transducer and activator of transcription |
| TOP2A | DNA topoisomerase II alpha |
| TP53 | tumor protein P53 |
| VEGF | vascular endothelial growth factors |
| VEGFR | vascular endothelial growth factor receptors |
| EGF | epidermal growth factors |
| EGFR | epidermal growth factor receptors |
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Zeyad Saeed Al-Shahrani, A.I.A.-R.; Amal Nasser Al-Madouj, M.S.H. Cancer Incidence Report Saudi Arabia; Saudi Health Council: Riyadh, Saudi Arabia, 2017. Available online: https://nhic.gov.sa/eServices/Documents/2014.pdf (accessed on 8 January 2021).
- El Zouhairi, M.; Charabaty, A.; Pishvaian, M.J. Molecularly targeted therapy for metastatic colon cancer: Proven treatments and promising new agents. Gastrointest. Cancer Res. 2011, 4, 15–21. [Google Scholar]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yin, Y.; Xu, S.J.; Chen, W.S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, G.; Febbraro, A.; Venditti, M.; Campidoglio, S.; Olivieri, N.; Raieta, K.; Parcesepe, P.; Imbriani, G.C.; Remo, A.; Pancione, M. Targeting angiogenesis and tumor microenvironment in metastatic colorectal cancer: Role of aflibercept. Gastroenterol. Res. Pract. 2014, 2014, 526178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, L.; Salem, M.E.; Mikhail, S. Biomarkers of Angiogenesis in Colorectal Cancer. Biomark. Cancer 2015, 7 (Suppl. 1), 13–19. [Google Scholar] [CrossRef]
- Hamada, T.; Nowak, J.A.; Ogino, S. PIK3CA mutation and colorectal cancer precision medicine. Oncotarget 2017, 8, 22305–22306. [Google Scholar] [CrossRef]
- Milano, G. PIK3CA mutations and specific treatment: Do not forget lessons from RAS mutations and EGFR targeting. Cancer Chemother. Pharmacol. 2020, 85, 473–474. [Google Scholar] [CrossRef]
- Lopez, A.; Harada, K.; Vasilakopoulou, M.; Shanbhag, N.; Ajani, J.A. Targeting Angiogenesis in Colorectal Carcinoma. Drugs 2019, 79, 63–74. [Google Scholar] [CrossRef]
- Vergoulidou, M. More than a Decade of Tyrosine Kinase Inhibitors in the Treatment of Solid Tumors: What We Have Learned and What the Future Holds. Biomark. Insights 2015, 10 (Suppl. 3), 33–40. [Google Scholar] [CrossRef]
- Estrada, C.C.; Maldonado, A.; Mallipattu, S.K. Therapeutic Inhibition of VEGF Signaling and Associated Nephrotoxicities. J. Am. Soc. Nephrol. 2019, 30, 187–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Aranda, M.; Redondo, M. Targeting Receptor Kinases in Colorectal Cancer. Cancers 2019, 11, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyabsi, M.; Alhumaid, A.; Allah-Bakhsh, H.; Alkelya, M.; Aziz, M.A. Colorectal cancer in Saudi Arabia as the proof-of-principle model for implementing strategies of predictive, preventive, and personalized medicine in healthcare. EPMA J. 2020, 11, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Eldai, H.; Periyasamy, S.; Al Qarni, S.; Al Rodayyan, M.; Muhammed Mustafa, S.; Deeb, A.; Al Sheikh, E.; Afzal, M.; Johani, M.; Yousef, Z.; et al. Novel genes associated with colorectal cancer are revealed by high resolution cytogenetic analysis in a patient specific manner. PLoS ONE 2013, 8, e76251. [Google Scholar]
- Alhadheq, A.M.; Purusottapatnam Shaik, J.; Alamri, A.; Aljebreen, A.M.; Alharbi, O.; Almadi, M.A.; Alhadeq, F.; Azzam, N.A.; Semlali, A.; Alanazi, M.; et al. The Effect of Poly(ADP-ribose) Polymerase-1 Gene 3’Untranslated Region Polymorphism in Colorectal Cancer Risk among Saudi Cohort. Dis. Markers 2016, 2016, 8289293. [Google Scholar] [CrossRef] [Green Version]
- Al-Kuraya, K.S.; Bavi, P.P.; Ezzat, A.A.; Al-Dayel, F.A.; Uddin, S.; Atizado, V.L.; Al-Jomah, N.A.; Amr, S.S.; Sheikh, S.S.; Sauter, G.; et al. Colorectal carcinoma from Saudi Arabia. Analysis of MLH-1, MSH-2 and p53 genes by immunohistochemistry and tissue microarray analysis. Saudi Med. J. 2006, 27, 323–328. [Google Scholar]
- Hu, T.; Li, Z.; Gao, C.Y.; Cho, C.H. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J. Gastroenterol. 2016, 22, 6876–6889. [Google Scholar] [CrossRef]
- Szakacs, G.; Annereau, J.P.; Lababidi, S.; Shankavaram, U.; Arciello, A.; Bussey, K.J.; Reinhold, W.; Guo, Y.; Kruh, G.D.; Reimers, M.; et al. Predicting drug sensitivity and resistance: Profiling ABC transporter genes in cancer cells. Cancer Cell 2004, 6, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Qutub, R.M.; Al-Ghafari, A.B.; Al Doghaither, H.A.; Omar, U.M.; Ghulam, J.M. Increased expressions of cellular ATP-binding cassette transporters may be a promising diagnostic marker for colorectal cancer. Saudi Med. J. 2020, 41, 834–840. [Google Scholar] [CrossRef]
- Al-Ghafari, A.B.; Qahtani, A.M.A.; Alturki, S.N.; Doghaither, H.A.A.; Elmorsy, E.M.; Tashkandi, H.M.; Abusanad, A.M.; Alkhayyat, S.S.; Omar, U.M.; Zeeneldin, A.A. Association between MDR1 polymorphisms and XELIRI and XELOX chemoresistance in Saudi patients with colorectal cancer. Oncol. Lett. 2020, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.M.; Roh, Y.J.; Kim, I.W.; Hasan, T.; Choi, M.G. Enhanced efficacy of photodynamic therapy by inhibiting ABCG2 in colon cancers. BMC Cancer 2015, 15, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aires, V.; Colin, D.J.; Doreau, A.; Di Pietro, A.; Heydel, J.M.; Artur, Y.; Latruffe, N.; Delmas, D. P-Glycoprotein 1 Affects Chemoactivities of Resveratrol against Human Colorectal Cancer Cells. Nutrients 2019, 11, 2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W.; Jung, K.H.; Byun, Y.; Lee, J.H.; Moon, S.H.; Cho, Y.S.; Lee, K.H. ATP-binding Cassette Transporters Substantially Reduce Estimates of ALDH-positive Cancer Cells based on Aldefluor and AldeRed588 Assays. Sci. Rep. 2019, 9, 6462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Chen, Z.; Zhu, Y.; Pan, Q.; Liu, Y.; Qi, X.; Jin, L.; Jin, J.; Ma, X.; Hua, D. Inhibition of transient receptor potential channel 5 reverses 5-Fluorouracil resistance in human colorectal cancer cells. J. Biol. Chem. 2015, 290, 448–456. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.A.; Mirza, A.A.; Hani, E.T.; Mirza, A.A.; Alsawaf, E.H. Colorectal Cancer: Molecular Classification And Clinical Application. World J. Res. Rev. 2016, 3, 5–9. [Google Scholar]
- García-Campos, M.A.; Espinal-Enríquez, J.; Hernández-Lemus, E. Pathway Analysis: State of the Art. Front. Physiol. 2015, 6, 383. [Google Scholar] [CrossRef] [Green Version]
- Hilhorst, M.H.; Houkes, L.; Korsten, H.; Mommersteeg, M.; Trapman, J.; Ruijtenbeek, R. Direct Detection of AKT/PKB Activity in a Pten Knock out Mouse Model Using Dynamic Peptide Microarrays; AACR: Philadelphia, PA, USA, 2011. [Google Scholar]
- Chirumamilla, C.S.; Fazil, M.; Perez-Novo, C.; Rangarajan, S.; de Wijn, R.; Ramireddy, P.; Verma, N.K.; Vanden Berghe, W. Profiling Activity of Cellular Kinases in Migrating T-Cells. Methods Mol. Biol. 2019, 1930, 99–113. [Google Scholar]
- Hilhorst, R.; Houkes, L.; Mommersteeg, M.; Musch, J.; van den Berg, A.; Ruijtenbeek, R. Peptide microarrays for profiling of serine/threonine kinase activity of recombinant kinases and lysates of cells and tissue samples. Methods Mol. Biol. 2013, 977, 259–271. [Google Scholar]
- Abdalla, A.N.; Qattan, A.; Malki, W.H.; Shahid, I.; Hossain, M.A.; Ahmed, M. Significance of Targeting VEGFR-2 and Cyclin D1 in Luminal-A Breast Cancer. Molecules 2020, 25, 4606. [Google Scholar] [CrossRef]
- Jin, H.; Dan, H.G.; Rao, G.-W. Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors. Heterocycl. Commun. 2018, 24, 1–10. [Google Scholar] [CrossRef]
- Solyanik, G.I. Quinazoline compounds for antitumor treatment. Exp. Oncol. 2019, 41, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.L.; Gupta, P.; Cui, Q.; Ashar, Y.V.; Wu, Z.X.; Zeng, L.; Lei, Z.N.; Teng, Q.X.; Ashby, C.R., Jr.; Guan, Y.; et al. Sapitinib Reverses Anticancer Drug Resistance in Colon Cancer Cells Overexpressing the ABCB1 Transporter. Front. Oncol. 2020, 10, 574861. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, H.M.; Abdalla, A.N.; Obaidullah, A.J.; Alanazi, M.M.; Almehizia, A.A.; Alanazi, M.G.; Ahmed, A.Y.; Alwassil, O.I.; Darwish, H.W.; Abdel-Aziz, A.A.; et al. Synthesis, cytotoxic evaluation, and molecular docking studies of novel quinazoline derivatives with benzenesulfonamide and anilide tails: Dual inhibitors of EGFR/HER2. Bioorg. Chem. 2020, 95, 103461. [Google Scholar] [CrossRef]
- Dussaq, A.; Anderson, J.C.; Willey, C.D.; Almeida, J.S. Mechanistic Parameterization of the Kinomic Signal in Peptide Arrays. J. Proteom. Bioinform. 2016, 9, 151–157. [Google Scholar] [CrossRef]
- Jean, S.; Kiger, A.A. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014, 127 Pt 5, 923–928. [Google Scholar] [CrossRef] [Green Version]
- Fransson, S.; Abel, F.; Kogner, P.; Martinsson, T.; Ejeskär, K. Stage-dependent expression of PI3K/Akt-pathway genes in neuroblastoma. Int. J. Oncol. 2013, 42, 609–616. [Google Scholar] [CrossRef]
- He, Q.; Xu, Q.; Wu, W.; Chen, L.; Sun, W.; Ying, J. Comparison of KRAS and PIK3CA gene status between primary tumors and paired metastases in colorectal cancer. Onco Targets Ther. 2016, 9, 2329–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Kuraya, K.S. KRAS and TP53 mutations in colorectal carcinoma. Saudi J. Gastroenterol. 2009, 15, 217–219. [Google Scholar] [CrossRef]
- Sanchez, V.E.; Nichols, C.; Kim, H.N.; Gang, E.J.; Kim, Y.M. Targeting PI3K Signaling in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2019, 20, 412. [Google Scholar] [CrossRef] [Green Version]
- The PI3Kα Inhibitor Alpelisib Has Activity in PIK3CA-altered Tumors. Cancer Discov. 2018, 8, 7. [CrossRef] [PubMed] [Green Version]
- Surawska, H.; Ma, P.C.; Salgia, R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev. 2004, 15, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Cortina, C.; Palomo-Ponce, S.; Iglesias, M.; Fernández-Masip, J.L.; Vivancos, A.; Whissell, G.; Humà, M.; Peiró, N.; Gallego, L.; Jonkheer, S.; et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat. Genet. 2007, 39, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Herath, N.I.; Boyd, A.W. The role of Eph receptors and ephrin ligands in colorectal cancer. Int. J. Cancer 2010, 126, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Baron, W.; Shattil, S.J.; ffrench-Constant, C. The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of alpha(v)beta3 integrins. EMBO J. 2002, 21, 1957–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldin, C.H.; Ostman, A.; Rönnstrand, L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta 1998, 1378, F79–F113. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox. Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Truong, T.H.; Garcia, F.J.; Homann, A.; Gupta, V.; Leonard, S.E.; Carroll, K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011, 8, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Lander, H.M.; Ogiste, J.S.; Teng, K.K.; Novogrodsky, A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. J. Biol. Chem. 1995, 270, 21195–21198. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Jiang, Y.L.; Lin, C.M.; Tsai, S.C.; Peng, S.F.; Fushiya, S.; Hour, M.J.; Yang, J.S. Dual inhibition of EGFR and c-Met kinase activation by MJ-56 reduces metastasis of HT29 human colorectal cancer cells. Int. J. Oncol. 2013, 43, 141–150. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, M.; Luo, C.C.; Wang, J.Q.; Zheng, N. Lactoferrin Exerts Antitumor Effects by Inhibiting Angiogenesis in a HT29 Human Colon Tumor Model. J. Agric. Food Chem. 2017, 65, 10464–10472. [Google Scholar] [CrossRef] [PubMed]
- SiShi Li, E.B. Lihua Wu, Jeffrey, D.; Bjorge, Donald, J.; Fujita, Shudong Zhu, EGFR and HER2 levels are frequently elevated in colon cancer cells. Discov. Rep. 2014, 1, 1–8. [Google Scholar]
- Dittmann, A.; Werner, T.; Chung, C.W.; Savitski, M.M.; Fälth Savitski, M.; Grandi, P.; Hopf, C.; Lindon, M.; Neubauer, G.; Prinjha, R.K.; et al. The commonly used PI3-kinase probe LY294002 is an inhibitor of BET bromodomains. ACS Chem. Biol. 2014, 9, 495–502. [Google Scholar] [CrossRef]
- Yang, S.Y.; Miah, A.; Sales, K.M.; Fuller, B.; Seifalian, A.M.; Winslet, M. Inhibition of the p38 MAPK pathway sensitises human colon cancer cells to 5-fluorouracil treatment. Int. J. Oncol. 2011, 38, 1695–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blain, S.W.; Scher, H.I.; Cordon-Cardo, C.; Koff, A. p27 as a target for cancer therapeutics. Cancer Cell 2003, 3, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Zirbes, T.K.; Baldus, S.E.; Moenig, S.P.; Nolden, S.; Kunze, D.; Shafizadeh, S.T.; Schneider, P.M.; Thiele, J.; Hoelscher, A.H.; Dienes, H.P. Prognostic impact of p21/waf1/cip1 in colorectal cancer. Int. J. Cancer 2000, 89, 14–18. [Google Scholar] [CrossRef]
- Donjerkovic, D.; Scott, D.W. Regulation of the G1 phase of the mammalian cell cycle. Cell Res. 2000, 10, 1–16. [Google Scholar] [CrossRef]
- Eid, S.Y.; Althubiti, M.A.; Abdallah, M.E.; Wink, M.; El-Readi, M.Z. The carotenoid fucoxanthin can sensitize multidrug resistant cancer cells to doxorubicin via induction of apoptosis, inhibition of multidrug resistance proteins and metabolic enzymes. Phytomedicine 2020, 77, 153280. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Abdallah, M.E.; Aslam, A.; Bader, A.; Vassallo, A.; Tommasi, N.; Malki, W.H.; Gouda, A.M.; Mukhtar, M.H.; El-Readi, M.Z.; et al. Synergistic Anti Leukemia Effect of a Novel Hsp90 and a Pan Cyclin Dependent Kinase Inhibitors. Molecules 2020, 25, 2220. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Shaheen, U.; Abdallah, Q.M.A.; Flamini, G.; Bkhaitan, M.M.; Abdelhady, M.I.S.; Ascrizzi, R.; Bader, A. Proapoptotic Activity of Achillea membranacea Essential Oil and Its Major Constituent 1,8-Cineole against A2780 Ovarian Cancer Cells. Molecules 2020, 25, 1582. [Google Scholar] [CrossRef] [Green Version]
- Chahrour, O.; Abdalla, A.; Lam, F.; Midgley, C.; Wang, S. Synthesis and biological evaluation of benzyl styrylsulfonyl derivatives as potent anticancer mitotic inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 3066–3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouda, A.M.; Abdelazeem, A.H.; Abdalla, A.N.; Ahmed, M. Pyrrolizine-5-carboxamides: Exploring the impact of various substituents on anti-inflammatory and anticancer activities. Acta Pharm. 2018, 68, 251–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, U.; Ragab, E.A.; Abdalla, A.N.; Bader, A. Triterpenoidal saponins from the fruits of Gleditsia caspica with proapoptotic properties. Phytochemistry 2018, 145, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Malki, W.H.; Gouda, A.M.; Ali, H.E.A.; Al-Rousan, R.; Samaha, D.; Abdalla, A.N.; Bustamante, J.; Abd Elmageed, Z.Y.; Ali, H.I. Structural-based design, synthesis, and antitumor activity of novel alloxazine analogues with potential selective kinase inhibition. Eur. J. Med. Chem. 2018, 152, 31–52. [Google Scholar] [CrossRef]













| Sex | na | Age | n | BMI b | n | CEA c | n | No. Lymph Nodes | n | T-stage | n | LVI d | n | KRAS | n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F e | 7 | 30–39 | 1 | 15–19 | 1 | 0–1 | 1 | 10–14 | 8 | 2 | 2 | Yes | 2 | Yes | 8 |
| M f | 3 | 40–49 | 1 | 20–24 | 0 | 2–3 | 5 | 15–19 | 1 | 3 | 6 | No | 8 | No | 2 |
| 50–59 | 4 | 25–29.9 | 6 | 4–5 | 1 | 20–24 | 1 | 4 | 2 | ||||||
| 60–69 | 2 | ≥30 | 3 | 6–7 | 3 | ||||||||||
| 70–79 | 2 |
| Enrichment by Pathway Maps | Total | p-Value | FDR ** | In Data | Network Objects from Active Data |
|---|---|---|---|---|---|
| PI3K/AKT pathway | 50 | 1.303 × 10−18 | 1.521 × 10−15 | 17 | BAD, NF-kB p52/p65, JAK1, NF-kB p50/p65, c-Raf-1, NF-kB p65/c-Rel, RelA (p65 NF-kB subunit), PI3K reg class IA (p85-alpha), Pyk2(FAK2), PKC-alpha, PDK (PDPK1), NF-kB, c-Src, NF-kB1 (p105), PI3K reg class IA (p85), CDK2, FAK1 |
| Inhibition of Ephrin receptors in colorectal cancer | 30 | 7.103 × 10−18 | 4.144 × 10−15 | 14 | Ephrin-B receptors, Ephrin-A receptors, Ephrin-A receptor 2, Ephrin-B receptor 3, c-Rel (NF-kB subunit), Ephrin-B receptor 4, Ephrin-B receptor 2, Ephrin-A receptor 1, Beta-catenin, Ephrin-A receptor 3, Ephrin-B receptor 1, Ephrin-A receptor 7, Paxillin, FAK1 |
| Development of growth factors in regulation of oligodendrocyte progenitor cell proliferation | 67 | 1.477 × 10−17 | 5.747 × 10−15 | 18 | EGFR, KV1.6, c-Raf-1, IGF-1 receptor, PDK (PDPK1), HGF receptor (Met), FGFR1, ErbB2, Vitronectin, FGFR3, PKC, PDGF-R-alpha, PI3K reg class IA (p85), Fyn, Lyn, PLC-gamma 1, PI3K reg class IA, TrkA |
| Oxidative stress ROS-mediated MAPK activation via canonical pathways | 60 | 4.446 × 10−17 | 1.297 × 10−14 | 17 | EGFR, ERK5 (MAPK7), CaMK II, JNK(MAPK8-10), c-Raf-1, JNK2(MAPK9), Pyk2(FAK2), CaMK II alpha, FGFR1, JAK2, SFK, CaMK II delta, c-Src, PDGF-R-beta, Fyn, PLC-gamma 1, JNK1(MAPK8) |
| Immune response M-CSF-receptor signaling pathway | 81 | 5.906 × 10−16 | 1.378 × 10−13 | 18 | YES, ERK5 (MAPK7), CaMK II, JAK1, c-Raf-1, M-CSF receptor, Hck, c-Cbl, Pyk2(FAK2), PDK (PDPK1), Beta-catenin, NF-kB, PLC-gamma, PKC, c-Src, PI3K reg class IA (p85), Fyn, p120GAP |
| Development EGFR signaling pathway | 71 | 1.017 × 10−15 | 1.978 × 10−13 | 17 | EGFR, JAK1, c-Raf-1, c-Cbl, JNK2(MAPK9), PKC-alpha, PDK (PDPK1), NF-kB, PKC-beta, JAK2, ErbB2, c-Src, PI3K reg class IA (p85), PLC-gamma 1, FAK1, JNK1(MAPK8), p120GAP |
| Development VEGF signaling via VEGFR2—generic cascades | 93 | 7.941 × 10−15 | 1.323 × 10−12 | 18 | NF-kB p50/p65, c-Raf-1, VEGFR-2, CREB1, Pyk2(FAK2), PKC-alpha, PDK (PDPK1), Beta-catenin, PKC-beta, Paxillin, PKC, c-Src, eNOS, Fyn, PLC-gamma 1, PI3K reg class IA, FAK1, p120GAP |
| Proliferative action of Gastrin in gastric cancer | 53 | 8.453 × 10−14 | 1.233 × 10−11 | 14 | EGFR, c-Raf-1, CREB1, PKC-alpha, PDK (PDPK1), Beta-catenin, PKC-beta, JAK2, PKC, c-Src, PI3K reg class IA (p85), cPKC (conventional), PLC-gamma 1, FAK1 |
| Development: The role of GDNF ligand family/RET receptor in cell survival, growth, and proliferation | 92 | 1.026 × 10−13 | 1.330 × 10−11 | 17 | c-Raf-1, JNK2(MAPK9), CREB1, RET, CaMK II alpha, PDK (PDPK1), ATF-1, NF-kB, CREM (activators), Paxillin, VEGFR-1, c-Src, PI3K reg class IA (p85), PLC-gamma 1, CDK2, FAK1, JNK1(MAPK8) |
| Immune response IL-4 signaling pathway | 94 | 1.490 × 10−13 | 1.738 × 10−11 | 17 | BAD, JNK(MAPK8-10), GSK3 alpha/beta, JAK1, NF-kB p50/p65, c-Raf-1, c-Cbl, c-Rel (NF-kB subunit), CREB1, NF-kB p50/RelB, PI3K reg class IA (p85-alpha), PDK (PDPK1), PLC-gamma, JAK2, c-Fes, PKC, PLC-gamma 1 |
| Drug(s) (Ratio) | HT29 | HT29-5FU | |||||
|---|---|---|---|---|---|---|---|
| IC50 | CI a | r b | IC50 | CI | r | FR c | |
| 5FU | 0.23 ± 0.04 | - | 0.97 | 68.12 ± 9.00 | - | 0.90 | - |
| LY294002 | 8.67 ± 0.70 | - | 0.81 | 30.56 ± 7.31 | - | 0.92 | - |
| HAA2020 | 3.75 ± 0.82 | - | 0.71 | 9.11 ± 1.99 | - | 0.89 | - |
| 5FU: LY294002 (1:1) | 0.40 ± 0.06 | 1.44 | 0.83 | 51.45 ± 7.31 | 33.12 | 0.93 | 1.3 |
| 5FU: HAA2020 (1:1) | 0.05 ± 0.00 | 0.10 | 0.88 | 9.01 ± 1.33 | 0.80 | 0.88 | 7.5 |
| LY294002:HAA2020 (1:1) | 0.95 ± 0.09 | 0.31 | 0.95 | 20.05 ± 4.11 | 2.46 | 0.87 | - |
| 5FU: LY294002: HAA2020 (1:1:1) | 0.09 ± 0.01 | 0.25 | 0.95 | 15.62 ± 2.27 | 12.21 | 0.70 | 4.3 |
| Drug(s) (Ratio) | HCT116 | HCT116-5FU | |||||
|---|---|---|---|---|---|---|---|
| IC50 | CI | r | IC50 | CI | r | FR | |
| 5FU | 0.19 ± 0.03 | - | 0.90 | 44.00 ± 5.10 | - | 0.93 | - |
| LY294002 | 11.54 ± 01.22 | - | 0.92 | 39.34 ± 5.12 | - | 0.96 | - |
| HAA2020 | 4.11 ± 0.50 | - | 0.98 | 13.33 ± 0.65 | - | 0.90 | - |
| 5FU: LY294002 (1:1) | 3.16 ± 0.67 | 12.87 | 0.91 | 40.23 ± 4.10 | 17.70 | 0.92 | 1.1 |
| 5FU: HAA2020 (1:1) | 0.15 ± 0.03 | 0.90 | 0.95 | 8.00 ± 1.12 | 0.95 | 0.90 | 5.5 |
| LY294002:HAA2020 (1:1) | 6.01 ± 0.89 | 5.11 | 0.89 | 30.88 ± 3.40 | 9.11 | 0.91 | - |
| 5FU: LY294002: HAA2020 (1:1:1) | 3.76 ± 0.41 | 2.20 | 0.96 | 25.11 ± 3.00 | 17.00 | 0.92 | 1.7 |
| Drug(s) (Ratio) | IC50 | SI d | |||
|---|---|---|---|---|---|
| MRC5 | HT29 | HT29-5FU | HCT116 | HCT116-5FU | |
| 5FU | 30.91 ± 4.22 | 134.4 | 0.4 | 162.6 | 0.7 |
| LY294002 | 28.65 ± 2.56 | 3.2 | 0.9 | 2.5 | 0.7 |
| HAA2020 | 19.44 ± 1.99 | 5.2 | 2.1 | 4.7 | 1.4 |
| 5FU: LY294002 (1:1) | 12.51 ± 1.40 | 31.2 | 0.2 | 3.9 | 0.3 |
| 5FU: HAA2020 (1:1) | 10.30 ± 0.78 | 206.0 | 1.1 | 68.6 | 1.2 |
| LY294002:HAA2020 (1:1) | 8.79 ± 1.34 | 9.6 | 0.4 | 1.5 | 0.3 |
| 5FU: LY294002: HAA2020 (1:1:1) | 5.40 ± 0.94 | 60.0 | 0.3 | 1.4 | 0.2 |
| Drug(s) (ratio) | IC50 | SI | |||
|---|---|---|---|---|---|
| HUVEC | HT29 | HT29-5FU | HCT116 | HCT116-5FU | |
| 5FU | 11.01 ± 1.09 | 55.0 | 0.2 | 57.9 | 0.3 |
| LY294002 | 9.02 ± 0.62 | 1.0 | 0.3 | 0.8 | 0.2 |
| HAA2020 | 32.00 ± 3.54 | 8.5 | 3.5 | 7.8 | 2.4 |
| 5FU: LY294002 (1:1) | 8.56 ± 0.71 | 21.4 | 0.2 | 2.7 | 0.2 |
| 5FU: HAA2020 (1:1) | 27.09 ± 3.00 | 541 | 3.0 | 180.6 | 3.4 |
| LY294002:HAA2020 (1:1) | 15.31 ± 2.11 | 16.1 | 0.8 | 2.5 | 0.5 |
| 5FU: LY294002: HAA2020 (1:1:1) | 3.11 ± 0.19 | 34.5 | 0.2 | 0.8 | 0.1 |
| Gene | Sequence | Gene | Sequence |
|---|---|---|---|
| GAPDH | F:AGGTCGGTGTGAACGGATTTG R:TGTAGACCATGTAGTTGAGGTCA | KRAS | F:CACTGTAATAATCCAGACTGTG R:CCCACCTATAATGGTGAATATC |
| ABCB1 | F:TGCTCAGACAGGATGTGAGTTG R:AATTACAGCAAGCCTGGAACC | ABCC1 | F:GCCAAGAAGGAGGAGACC R:AGGAAGATGCTGAGGAAGG |
| ABCG2 | F:TATAGCTCAGATCATTGTCACAGTC R:GTTGGTCGTCAGGAAGAAGAG | PIK3CA | F:AGACACAAAACAGGCTCAGGA R:TTGAGAGAAAAACTGATAT ATTAAATGAC |
| AKT | F:GTGGCAAGATGTGTATGAG R:CTGGCTGAGTAGGAGAAC | MAPK7 | F:ACCGAAGGACGCTTGTTAG R:AGCAGCAGCAGAACCAAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, A.N.; Malki, W.H.; Qattan, A.; Shahid, I.; Hossain, M.A.; Ahmed, M. Chemosensitization of HT29 and HT29-5FU Cell Lines by a Combination of a Multi-Tyrosine Kinase Inhibitor and 5FU Downregulates ABCC1 and Inhibits PIK3CA in Light of Their Importance in Saudi Colorectal Cancer. Molecules 2021, 26, 334. https://doi.org/10.3390/molecules26020334
Abdalla AN, Malki WH, Qattan A, Shahid I, Hossain MA, Ahmed M. Chemosensitization of HT29 and HT29-5FU Cell Lines by a Combination of a Multi-Tyrosine Kinase Inhibitor and 5FU Downregulates ABCC1 and Inhibits PIK3CA in Light of Their Importance in Saudi Colorectal Cancer. Molecules. 2021; 26(2):334. https://doi.org/10.3390/molecules26020334
Chicago/Turabian StyleAbdalla, Ashraf N., Waleed H. Malki, Amal Qattan, Imran Shahid, Mohammad Akbar Hossain, and Muhammad Ahmed. 2021. "Chemosensitization of HT29 and HT29-5FU Cell Lines by a Combination of a Multi-Tyrosine Kinase Inhibitor and 5FU Downregulates ABCC1 and Inhibits PIK3CA in Light of Their Importance in Saudi Colorectal Cancer" Molecules 26, no. 2: 334. https://doi.org/10.3390/molecules26020334
APA StyleAbdalla, A. N., Malki, W. H., Qattan, A., Shahid, I., Hossain, M. A., & Ahmed, M. (2021). Chemosensitization of HT29 and HT29-5FU Cell Lines by a Combination of a Multi-Tyrosine Kinase Inhibitor and 5FU Downregulates ABCC1 and Inhibits PIK3CA in Light of Their Importance in Saudi Colorectal Cancer. Molecules, 26(2), 334. https://doi.org/10.3390/molecules26020334