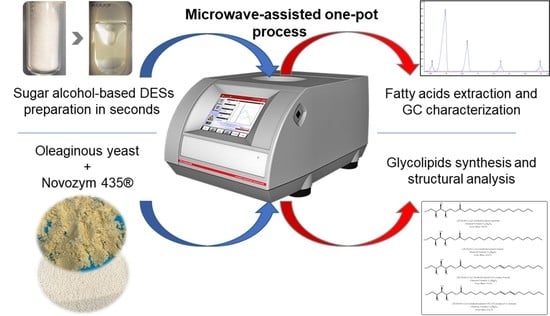
Microwave-Assisted One-Pot Lipid Extraction and Glycolipid Production from Oleaginous Yeast Saitozyma podzolica in Sugar Alcohol-Based Media
Abstract
:1. Introduction
2. Results
2.1. Comparative Study on the Production Time of Common and Sugar-Alcohol Based DESs
2.2. Post-Reaction Thin Layer Chromatography (TLC) of Glycolipids- and Non Glycolipids-Containing Mixtures
2.3. Comparison of Extracted Whole Cell Lipid and Esterified Fatty Acids
2.4. Profiling of Extracted and Esterified Lipids
2.5. Structural Elucidation Using Spectroscopic and Spectrometric Methods
3. Discussion
3.1. Microwave and DES Technologies: An Optimal Match?
3.2. Unconventional Media for Lipid Extraction and Subsequent Production of Glycolipids
4. Materials and Methods
4.1. Materials
4.2. Microorganisms
4.3. DES Preparation with Microwave Dielectric Heating and Conventional Convective Heating
4.4. Production of Single Cell Oil in Bioreactors
4.5. Microwave Processing of the Oleaginous Biomass
4.6. DownStream Processing (DSP) and Flash Chromatorgaphy Purification of the Reaction’s Crude
4.7. Folch Extraction and Direct Acidic Transesterification of the Biomass
4.8. Acidic Transesterification to Fatty Acid Methyl Esters (FAMEs) of the Lipid Fraction
4.9. GC Analysis of Fatty Acid Methyl Esters (FAMEs)
4.10. Thin Layer Chromatography (TLC) Analysis of Reaction Mixtures
4.11. Spectroscopic and Spectrometric Methods for Structural Eluciation of Glycolipids
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| Xylitol-Based DES Process | ||
|---|---|---|
| Molecule | m/z | Adducts |
| Xylitol palmitate (XP) | 391.284 | [XP+H]+ |
| 409.439 | [XP+NH4]+ | |
| Xylitol oleate (XO) | 399.346 | [XO+H]+-H2O |
| 417.321 | [XO+H]+ | |
| 434.33 | [XO+NH4]+ | |
| 439.450 | [XO+Na]+ | |
| Xylitol Linoleate (XL) | 396.427 | [XL+H]+-H2O |
| 414.962 | [XL+H]+ | |
| 430.976 | [XL+NH4]+ | |
| 437.434 | [XL+Na]+ | |
| Xylitol Stearate (XS) | 401.341 | [XS+H]+-H2O |
| 419.315 | [XS+H]+ | |
| Sorbitol-Based DES Process | ||
| Molecule | m/z | Adducts |
| Sorbitol palmitate (SP) | 387.235 | [SP+H]+-H2O |
| 421.204 | [SP+H]+ | |
| 443.232 | [SP+Na]+ | |
| 437.235 | [SP+NH4]+ | |
| Sorbitol oleate (SO) | 393.209 | [SO+H]+-3H2O |
| 447.292 | [SO+H]+ | |
| 465.230 | [SO+NH4]+ | |
| Sorbitol linoleate (SL) | 391.283 | [SL+H]+-3H2O |
| 409.183 | [SL+H]+-2H2O | |
| 445.276 | [SL+H]+ | |
| 462.275 | [SL+NH4]+ | |
| Sorbitol stearate (SS) | 431.261 | [SS+H]+-H2O |
| 449.271 | [SS+H]+ | |
| 473.344 | [SS+Na]+ | |
References
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; de Jesús Cázares-Marinero, J. Biosurfactants: The Green Generation of Speciality Chemicals and Potential Production Using Solid-State Fermentation (SSF) Technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.; Kotta, S.; Jijith, U.S.; Aravind, A.; Abu Tahir, M.; Manju, C.S.; Gangadharappa, H.V. Surfactant-Based Prophylaxis and Therapy against COVID-19: A Possibility. Med. Hypotheses 2020, 143, 110081. [Google Scholar] [CrossRef] [PubMed]
- Gallou, F.; Isley, N.A.; Ganic, A.; Onken, U.; Parmentier, M. Surfactant Technology Applied toward an Active Pharmaceutical Ingredient: More than a Simple Green Chemistry Advance. Green Chem. 2016, 18, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Baccile, N.; Nassif, N.; Malfatti, L.; Bogaert, I.N.A.V.; Soetaert, W.; Pehau-Arnaudet, G.; Babonneau, F. Sophorolipids: A Yeast-Derived Glycolipid as Greener Structure Directing Agents for Self-Assembled Nanomaterials. Green Chem. 2010, 12, 1564–1567. [Google Scholar] [CrossRef] [Green Version]
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel Characteristics of Sophorolipids, Yeast Glycolipid Biosurfactants, as Biodegradable Low-Foaming Surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef]
- González-Rivera, J.; Husanu, E.; Mero, A.; Ferrari, C.; Duce, C.; Tinè, M.R.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Insights into Microwave Heating Response and Thermal Decomposition Behavior of Deep Eutectic Solvents. J. Mol. Liq. 2020, 300, 112357. [Google Scholar] [CrossRef]
- Davies, E.; Deutz, P.; Zein, S.H. Single-Step Extraction-Esterification Process to Produce Biodiesel from Palm Oil Mill Effluent (POME) Using Microwave Heating: A Circular Economy Approach to Making Use of a Difficult Waste Product. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Siebenhaller, S.; Kirchhoff, J.; Kirschhöfer, F.; Brenner-Weiß, G.; Muhle-Goll, C.; Luy, B.; Haitz, F.; Hahn, T.; Zibek, S.; Syldatk, C.; et al. Integrated Process for the Enzymatic Production of Fatty Acid Sugar Esters Completely Based on Lignocellulosic Substrates. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Gorte, O.; Hollenbach, R.; Papachristou, I.; Steinweg, C.; Silve, A.; Frey, W.; Syldatk, C.; Ochsenreither, K. Evaluation of Downstream Processing, Extraction, and Quantification Strategies for Single Cell Oil Produced by the Oleaginous Yeasts Saitozyma Podzolica DSM 27192 and Apiotrichum Porosum DSM 27194. Front. Bioeng. Biotechnol. 2020, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production Strategies and Applications of Microbial Single Cell Oils. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Liauw, M.Y.; Natan, F.A.; Widiyanti, P.; Ikasari, D.; Indraswati, N.; Soetaredjo, F.E. Extraction of Neem Oil (Azadirachta Indica A. Juss) Using N-Hexane and Ethanol: Studies of Oil Quality, Kinetic and Thermodynamic. ARPN J. Eng. Appl. Sci. 2008, 3, 49–54. [Google Scholar]
- Dallinger, D.; Kappe, C.O. Microwave-Assisted Synthesis in Water as Solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef]
- Microwave Theory. In Microwaves in Organic and Medicinal Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 9–39. ISBN 978-3-527-64782-8.
- LidstroÈm, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave Assisted Organic SynthesisÐa Review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Nie, J.; Yu, G.; Song, Z.; Wang, X.; Li, Z.; She, Y.; Lee, M. Microwave-Assisted Deep Eutectic Solvent Extraction Coupled with Headspace Solid-Phase Microextraction Followed by GC-MS for the Analysis of Volatile Compounds from Tobacco. Anal. Methods 2017, 9, 856–863. [Google Scholar] [CrossRef]
- De la Hoz, A.; Díaz-Ortiz, Á.; Moreno, A. Microwaves in Organic Synthesis. Thermal and Non-Thermal Microwave Effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Herrero, M.A.; Kremsner, J.M.; Kappe, C.O. Nonthermal Microwave Effects Revisited: On the Importance of Internal Temperature Monitoring and Agitation in Microwave Chemistry. J. Org. Chem. 2008, 73, 36–47. [Google Scholar] [CrossRef]
- Tasei, Y.; Mijiddorj, B.; Fujito, T.; Kawamura, I.; Ueda, K.; Naito, A. Thermal and Nonthermal Microwave Effects of Ethanol and Hexane-Mixed Solution as Revealed by In Situ Microwave Irradiation Nuclear Magnetic Resonance Spectroscopy and Molecular Dynamics Simulation. J. Phys. Chem. B 2020, 124, 9615–9624. [Google Scholar] [CrossRef]
- Kubo, M.T.; Siguemoto, É.S.; Funcia, E.S.; Augusto, P.E.; Curet, S.; Boillereaux, L.; Sastry, S.K.; Gut, J.A. Non-Thermal Effects of Microwave and Ohmic Processing on Microbial and Enzyme Inactivation: A Critical Review. Curr. Opin. Food Sci. 2020, 35, 36–48. [Google Scholar] [CrossRef]
- Coultate, T.P. Polysaccharides. In Food; RSC Publishing: Cambridge, UK, 2001; pp. 41–72. ISBN 978-1-84973-880-4. [Google Scholar]
- Jiang, B.; Liu, Y.; Bhandari, B.; Zhou, W. Impact of Caramelization on the Glass Transition Temperature of Several Caramelized Sugars. Part I: Chemical Analyses. J. Agric. Food Chem. 2008, 56, 5138–5147. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, Y.M.; Al-Wahaibi, T.; Hashim, M.A. Glucose-Based Deep Eutectic Solvents: Physical Properties. J. Mol. Liq. 2013, 178, 137–141. [Google Scholar] [CrossRef]
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents from Choline Chloride and Betaine—Physicochemical Properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-Dependent Extraction of Flavonoids from Citrus Peel Waste Using a Tailor-Made Deep Eutectic Solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef]
- Sathish, A.; Smith, B.R.; Sims, R.C. Effect of Moisture on in Situ Transesterification of Microalgae for Biodiesel Production. J. Chem. Technol. Biotechnol. 2014, 89, 137–142. [Google Scholar] [CrossRef]
- Yousuf, A.; Khan, M.R.; Islam, M.A.; Wahid, Z.A.; Pirozzi, D. Technical Difficulties and Solutions of Direct Transesterification Process of Microbial Oil for Biodiesel Synthesis. Biotechnol. Lett. 2016. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.; Yang, F.; Hu, C.; Shen, H.; Zhao, Z.K. Enzyme-Assisted Extraction of Lipids Directly from the Culture of the Oleaginous Yeast Rhodosporidium Toruloides. Bioresour. Technol. 2012, 111, 378–382. [Google Scholar] [CrossRef]
- Khoomrung, S.; Chumnanpuen, P.; Jansa-Ard, S.; Ståhlman, M.; Nookaew, I.; Borén, J.; Nielsen, J. Rapid Quantification of Yeast Lipid Using Microwave-Assisted Total Lipid Extraction and HPLC-CAD. Anal. Chem. 2013, 85, 4912–4919. [Google Scholar] [CrossRef]
- Klei, I.V.D.; Veenhuis, M.; Brul, S.; Klis, F.M.; Groot, P.W.J.D.; Müller, W.H.; van Driel, K.G.A.; Boekhout, T. Cytology, Cell Walls and Septa: A Summary of Yeast Cell Biology from a Phylogenetic Perspective. Yeasts 2011, 111–128. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of Several Methods for Effective Lipid Extraction from Microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, E.; Cravotto, G.; Galletti, P.; Grillo, G.; Mazzotti, M.; Sacchetti, G.; Samorì, C.; Tabasso, S.; Tacchini, M.; Tagliavini, E. Enhanced and Selective Lipid Extraction from the Microalga P. Tricornutum by Dimethyl Carbonate and Supercritical CO2 Using Deep Eutectic Solvents and Microwaves as Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 8316–8322. [Google Scholar] [CrossRef]
- Martins, A.B.; Graebin, N.G.; Lorenzoni, A.S.G.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Rapid and High Yields of Synthesis of Butyl Acetate Catalyzed by Novozym 435: Reaction Optimization by Response Surface Methodology. Process Biochem. 2011, 46, 2311–2316. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “Perfect” Lipase Immobilized Biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Lieu, T.; Yusup, S.; Moniruzzaman, M. Kinetic Study on Microwave-Assisted Esterification of Free Fatty Acids Derived from Ceiba Pentandra Seed Oil. Bioresour. Technol. 2016, 211, 248–256. [Google Scholar] [CrossRef]
- Reyes, H.R.; Hill, C.G. Kinetic Modeling of Interesterification Reactions Catalyzed by Immobilized Lipase. Biotechnol. Bioeng. 1994, 43, 171–182. [Google Scholar] [CrossRef]
- Keramat, M.; Golmakani, M.-T.; Durand, E.; Villeneuve, P.; Hosseini, S.M.H. Auto-Catalytic Production of Eugenyl Acetate and Eugenyl Butyrate Using Microwave Radiation: A Kinetic and Mechanism-Related Approach. J. Chem. Technol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Choi, Y.-K.; Park, J.; Lee, S.; Yang, Y.-H.; Kim, H.J.; Park, T.-J.; Kim, Y.H.; Lee, S.H. Ionic Liquid-Mediated Extraction of Lipids from Algal Biomass. Bioresour. Technol. 2012, 109, 312–315. [Google Scholar] [CrossRef]
- Ganske, F.; Bornscheuer, U.T. Lipase-Catalyzed Glucose Fatty Acid Ester Synthesis in Ionic Liquids. Org. Lett. 2005, 7, 3097–3098. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Landeka Dragičević, T.; Radojčić Redovniković, I. Evaluation of Toxicity and Biodegradability of Choline Chloride Based Deep Eutectic Solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Lecomte, J.; Baréa, B.; Piombo, G.; Dubreucq, E.; Villeneuve, P. Evaluation of Deep Eutectic Solvents as New Media for Candida Antarctica B Lipase Catalyzed Reactions. Process Biochem. 2012, 47, 2081–2089. [Google Scholar] [CrossRef]
- Bermúdez, J.M.; Beneroso, D.; Rey-Raap, N.; Arenillas, A.; Menéndez, J.A. Energy Consumption Estimation in the Scaling-up of Microwave Heating Processes. Chem. Eng. Process. Process Intensif. 2015, 95, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pöhnlein, M.; Hausmann, R.; Lang, S.; Syldatk, C. Enzymatic Synthesis and Modification of Surface-Active Glycolipids: Enz Syn and Mod of Glycolipids. Eur. J. Lipid Sci. Technol. 2015, 117, 145–155. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Yamane, T. Fatty Acid Vinyl Esters as Acylating Agents: A New Method for the Enzymatic Synthesis of Monoacylglycerols. J. Am. Oil Chem. Soc. 1995, 72, 193–197. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Natural Surfactants Used in Cosmetics: Glycolipids. Int. J. Cosmet. Sci. 2009, 31, 255–261. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty Acid Composition of Black Soldier Fly Larvae (Hermetia illucens)—Possibilities and Limitations for Modification through Diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Hoc, B.; Genva, M.; Fauconnier, M.-L.; Lognay, G.; Francis, F.; Caparros Megido, R. About Lipid Metabolism in Hermetia Illucens (L. 1758): On the Origin of Fatty Acids in Prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef]
- Szűts, A.; Szabó-Révész, P. Sucrose Esters as Natural Surfactants in Drug Delivery Systems—A Mini-Review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef]
- Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant—Hepatoprotective Interplay. Pharmaceutics 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlson, P.; Doenecke, D.; Koolman, J.; Fuchs, G.; Gerok, W. Karlsons Biochemie und Pathobiochemie; Thieme: Stuttgart, Germany, 2005; ISBN 978-3-13-357815-8. [Google Scholar]
- Arcens, D.; Grau, E.; Grelier, S.; Cramail, H.; Peruch, F. Impact of Fatty Acid Structure on CALB-Catalyzed Esterification of Glucose. Eur. J. Lipid Sci. Technol. 2020, 122, 1900294. [Google Scholar] [CrossRef] [Green Version]
- Grüninger, J.; Delavault, A.; Ochsenreither, K. Enzymatic Glycolipid Surfactant Synthesis from Renewables. Process Biochem. 2019, 87, 45–54. [Google Scholar] [CrossRef]
- Schulze, I.; Hansen, S.; Großhans, S.; Rudszuck, T.; Ochsenreither, K.; Syldatk, C.; Neumann, A. Characterization of Newly Isolated Oleaginous Yeasts—Cryptococcus Podzolicus, Trichosporon Porosum and Pichia Segobiensis. AMB Express 2014, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliyu, H.; Gorte, O.; Neumann, A.; Ochsenreither, K. Draft Genome Sequence of the Oleaginous Yeast Saitozyma Podzolica (Syn. Cryptococcus Podzolicus) DSM 27192. Microbiol. Resour. Announc. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Pöhnlein, M.; Ulrich, J.; Kirschhöfer, F.; Nusser, M.; Muhle-Goll, C.; Kannengiesser, B.; Brenner-Weiß, G.; Luy, B.; Liese, A.; Syldatk, C.; et al. Lipase-Catalyzed Synthesis of Glucose-6-O-Hexanoate in Deep Eutectic Solvents. Eur. J. Lipid Sci. Technol. 2015, 117, 161–166. [Google Scholar] [CrossRef]





| DES Number/Name | HBD | mR [mol] | mHBD [g] | HBA | mR [mol] | mHBA [g] | mR [mol] | mwater [g] | wt % Water |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Arabinose | 1 | 6.45 | ChCl | 1 | 6.00 | 0.8 | 0.62 | 5 |
| 2 | Glucose | 1 | 5.00 | ChCl | 2 | 9.69 | 2.50 | 1.25 | 9 |
| 3 | Glycerol | 2 | 7.86 | Betaine | 1 | 5.00 | 1.00 | 0.77 | 6 |
| 4 | Urea | 2 | 4.30 | ChCl | 1 | 5.00 | - | - | - |
| 5 | Glycerol | 2 | 6.60 | ChCl | 1 | 5.00 | - | - | - |
| 6 | 1,2-Propanediol | 1 | 2.54 | ChCl | 1 | 4.65 | 1.00 | 0.60 | 8 |
| 7 | 1,4-Butanediol | 4 | 6.15 | Betaine | 1 | 2.00 | 1.00 | 0.31 | 4 |
| 8 | Saccharose | 1 | 5.00 | ChCl | 4 | 8.16 | 4.00 | 1.05 | 8 |
| Xylit | Xylitol | 1 | 5.45 | ChCl | 1 | 5.00 | 0.8 | 0.52 | 5 |
| Sorbit | Sorbitol | 1 | 6.53 | ChCl | 1 | 5.00 | 0.9 | 0.58 | 5 |
| Condition | DES | AcDES | DES + Lipase | AcDES + Lipase | FE | DT | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sugar Alchohol | Sorbitol | Xylitol | Sorbitol | Xylitol | Sorbitol | Xylitol | Sorbitol | Xylitol | - | - |
| Extracted whole lipids ** (total amount (mg)) | 32 ± 1 | 50 ± 2 | 90 ± 1 | 105 ± 1.5 | 35 ± 1 | 25 ± 1 | 63 ± 1 | 68 ± 3.3 | 43 ± 1 | - |
| Extracted FAMEs ** (total amount (mg)) | 14 ± 1 | 10 ± 2 | 45 ± 3 | 68 ± 3 | 2.4 ± 0.1 | 10 ± 1 | 40 ± 1 | 35 ± 1 | 27 ± 1 | 5 ± 1 *** |
| Glycolipid quantity (total amount (mg)) | - | - | T * | T * | - | - | ~15 | ~20 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delavault, A.; Ochs, K.; Gorte, O.; Syldatk, C.; Durand, E.; Ochsenreither, K. Microwave-Assisted One-Pot Lipid Extraction and Glycolipid Production from Oleaginous Yeast Saitozyma podzolica in Sugar Alcohol-Based Media. Molecules 2021, 26, 470. https://doi.org/10.3390/molecules26020470
Delavault A, Ochs K, Gorte O, Syldatk C, Durand E, Ochsenreither K. Microwave-Assisted One-Pot Lipid Extraction and Glycolipid Production from Oleaginous Yeast Saitozyma podzolica in Sugar Alcohol-Based Media. Molecules. 2021; 26(2):470. https://doi.org/10.3390/molecules26020470
Chicago/Turabian StyleDelavault, André, Katarina Ochs, Olga Gorte, Christoph Syldatk, Erwann Durand, and Katrin Ochsenreither. 2021. "Microwave-Assisted One-Pot Lipid Extraction and Glycolipid Production from Oleaginous Yeast Saitozyma podzolica in Sugar Alcohol-Based Media" Molecules 26, no. 2: 470. https://doi.org/10.3390/molecules26020470
APA StyleDelavault, A., Ochs, K., Gorte, O., Syldatk, C., Durand, E., & Ochsenreither, K. (2021). Microwave-Assisted One-Pot Lipid Extraction and Glycolipid Production from Oleaginous Yeast Saitozyma podzolica in Sugar Alcohol-Based Media. Molecules, 26(2), 470. https://doi.org/10.3390/molecules26020470