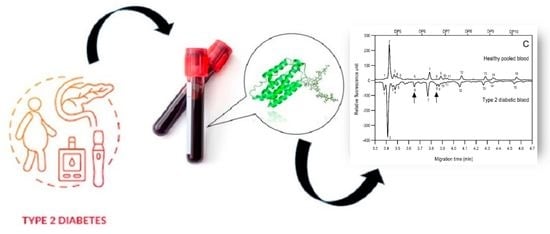
N-Glycosylation Profiling of Human Blood in Type 2 Diabetes by Capillary Electrophoresis: A Preliminary Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. N-Glycan Sample Preparation from Blood
4.3. Capillary Electrophoresis
4.4. Clinical Patient Information
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Unnikrishnan, R.; Anjana, R.M.; Mohan, V. Diabetes mellitus and its complications in India. Nat. Rev. Endocrinol. 2016, 12, 357–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmers, R.F.H.; Vilaj, M.; Urda, D.; Agakov, F.; Šimurina, M.; Klaric, L.; Rudan, I.; Campbell, H.; Hayward, C.; Wilson, J.F.; et al. IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2240–2249. [Google Scholar] [CrossRef]
- Morris, A.P.; Voight, B.F.; Teslovich, T.M.; Ferreira, T.; Segrè, A.V.; Steinthorsdottir, V.; Strawbridge, R.J.; Khan, H.; Grallert, H.; Mahajan, A.; et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 2012, 44, 981–990. [Google Scholar] [CrossRef]
- Lauc, G.; Pezer, M.; Rudan, I.; Campbell, H. Mechanisms of disease: The human N-glycome. Biochim. Biophys. Acta 2016, 1860, 1574–1582. [Google Scholar] [CrossRef] [Green Version]
- Gornik, O.; Pavić, T.; Lauc, G. Alternative glycosylation modulates function of IgG and other proteins—Implications on evolution and disease. Biochim. Biophys. Acta 2012, 1820, 1318–1326. [Google Scholar] [CrossRef]
- Reider, B.; Jarvas, G.; Krenkova, J.; Guttman, A. Separation based characterization methods for the N-glycosylation analysis of prostate-specific antigen. J. Pharm. Biomed. Anal. 2020, 194, 113797. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, B.; Járvás, G.; Farkas, A.; Szigeti, M.; Kovács, Z.; Kun, R.; Szabó, M.; Csánky, E.; Guttman, A. Comparative analysis of the human serum N-glycome in lung cancer, COPD and their comorbidity using capillary electrophoresis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1137, 121913. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Munkley, J. Glycans as Biomarkers in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1389. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Zhao, J.; Dong, X.; Banazadeh, A.; Huang, Y.; Hussien, A.; Mechref, Y. Clinical application of quantitative glycomics. Expert Rev. Proteomics 2018, 15, 1007–1031. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Liu, D.; Tao, L.; Zhang, J.; Liang, B.; Liu, X.; Wang, X.; Li, X.; Wang, Y.; et al. IgG Glycosylation Profile and the Glycan Score Are Associated with Type 2 Diabetes in Independent Chinese Populations: A Case-Control Study. J. Diabetes Res. 2020, 2020, 5041346. [Google Scholar] [CrossRef]
- Bermingham, M.L.; Colombo, M.; McGurnaghan, S.J.; Blackbourn, L.A.K.; Vučković, F.; Pučić Baković, M.; Trbojević-Akmačić, I.; Lauc, G.; Agakov, F.; Agakova, A.S.; et al. N-Glycan Profile and Kidney Disease in Type 1 Diabetes. Diabetes Care 2018, 41, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Uh, H.-W.; Beekman, M.; Koeleman, C.A.M.; Hokke, C.H.; Westendorp, R.G.J.; Wuhrer, M.; Houwing-Duistermaat, J.J.; Slagboom, P.E.; Deelder, A.M. Decreased levels of bisecting GlcNAc glycoforms of IgG are associated with human longevity. PLoS ONE 2010, 5, e12566. [Google Scholar] [CrossRef] [Green Version]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [Green Version]
- Pucić, M.; Knezević, A.; Vidic, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Supraha-Goreta, S.; Wormald, M.R.; Redzić, I.; et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteom. 2011, 10, M111.010090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knezevic, A.; Gornik, O.; Polasek, O.; Pucic, M.; Redzic, I.; Novokmet, M.; Rudd, P.M.; Wright, A.F.; Campbell, H.; Rudan, I.; et al. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology 2010, 20, 959–969. [Google Scholar] [CrossRef] [Green Version]
- Gudelj, I.; Keser, T.; Vučković, F.; Škaro, V.; Goreta, S.Š.; Pavić, T.; Dumić, J.; Primorac, D.; Lauc, G.; Gornik, O. Estimation of human age using N-glycan profiles from bloodstains. Int. J. Leg. Med. 2015, 129, 955–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adua, E.; Memarian, E.; Afrifa-Yamoah, E.; Russell, A.; Trbojević-Akmačić, I.; Gudelj, I.; Jurić, J.; Roberts, P.; Lauc, G.; Wang, W. N-glycosylation profiling of Type 2 diabetes mellitus from baseline to follow-up: An observational study in a Ghanaian population. Biomark. Med. 2021, 15, 467–480. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Asnin, L. Chiral separation of quinolones by liquid chromatography and capillary electrophoresis. J. Sep. Sci. 2017, 40, 2863–2882. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Yao, L.; Wang, C.; Van Schepdael, A. Recent advances in vitamins analysis by capillary electrophoresis. J. Pharm. Biomed. Anal. 2018, 147, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Timerbaev, A.R. Capillary electrophoresis of inorganic ions: An update. Electrophoresis 2004, 25, 4008–4031. [Google Scholar] [CrossRef]
- Laube, H.; Boden, J.; Schneider, R. Capillary electrophoresis method for the analysis of organic acids and amino acids in the presence of strongly alternating concentrations of aqueous lactic acid. Bioprocess Biosyst. Eng. 2017, 40, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Morani, M.; Taverna, M.; Mai, T.D. A fresh look into background electrolyte selection for capillary electrophoresis-laser induced fluorescence of peptides and proteins. Electrophoresis 2019, 40, 2618–2624. [Google Scholar] [CrossRef]
- Adamczyk, B.; Tharmalingam, T.; Rudd, P.M. Glycans as cancer biomarkers. Biochim. Biophys. Acta-Gen. Subj. 2012, 1820, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Le, A.T.H.; Krylova, S.M.; Krylov, S.N. Determination of the Equilibrium Constant and Rate Constant of Protein-Oligonucleotide Complex Dissociation under the Conditions of Ideal-Filter Capillary Electrophoresis. Anal. Chem. 2019, 91, 8532–8539. [Google Scholar] [CrossRef]
- Smith, A.; Nelson, R.J. Capillary electrophoresis of DNA. Curr. Protoc. Nucleic Acid Chem. 2003. Chapter 10, Unit 10.9. [Google Scholar] [CrossRef]
- Kitagawa, F.; Otsuka, K. Recent progress in capillary electrophoretic analysis of amino acid enantiomers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 3078–3095. [Google Scholar] [CrossRef]
- Gattu, S.; Crihfield, C.L.; Lu, G.; Bwanali, L.; Veltri, L.M.; Holland, L.A. Advances in enzyme substrate analysis with capillary electrophoresis. Methods 2018, 146, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Tharmalingam-Jaikaran, T.; Schomberg, M.; Szekrényes, Á.; Kelly, R.M.; Karlsson, N.G.; Guttman, A.; Rudd, P.M. Comparison of separation techniques for the elucidation of IgG N-glycans pooled from healthy mammalian species. Carbohydr. Res. 2014, 389, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Helander, A.; Wielders, J.P.M.; Te Stroet, R.; Bergström, J.P. Comparison of HPLC and capillary electrophoresis for confirmatory testing of the alcohol misuse marker carbohydrate-deficient transferrin. Clin. Chem. 2005, 51, 1528–1531. [Google Scholar] [CrossRef] [Green Version]
- Jarvas, G.; Szigeti, M.; Campbell, M.P.; Guttman, A. Expanding the capillary electrophoresis-based glucose unit database of the GUcal app. Glycobiology 2020, 30, 362–364. [Google Scholar] [CrossRef]
- Royle, L.; Campbell, M.P.; Radcliffe, C.M.; White, D.M.; Harvey, D.J.; Abrahams, J.L.; Kim, Y.-G.; Henry, G.W.; Shadick, N.A.; Weinblatt, M.E.; et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal. Biochem. 2008, 376, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J.; Merry, A.H.; Royle, L.; Campbell, M.P.; Rudd, P.M. Symbol nomenclature for representing glycan structures: Extension to cover different carbohydrate types. Proteomics 2011, 11, 4291–4295. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schisterman, E.F.; Vexler, A. To pool or not to pool, from whether to when: Applications of pooling to biospecimens subject to a limit of detection. Paediatr. Perinat. Epidemiol. 2008, 22, 486–496. [Google Scholar] [CrossRef] [Green Version]
- Reider, B.; Szigeti, M.; Guttman, A. Evaporative fluorophore labeling of carbohydrates via reductive amination. Talanta 2018, 185, 365–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szigeti, M.; Guttman, A. High-Throughput N-Glycan Analysis with Rapid Magnetic Bead-Based Sample Preparation. In High-Throughput Glycomics and Glycoproteomics; Humana Press: New York, NY, USA, 2017; pp. 265–272. [Google Scholar]
- Szigeti, M.; Guttman, A. Automated N-Glycosylation Sequencing of Biopharmaceuticals by Capillary Electrophoresis. Sci. Rep. 2017, 7, 11663. [Google Scholar] [CrossRef]



 Sialic acid (N-acetylneuraminic acid);
Sialic acid (N-acetylneuraminic acid);
 Galactose;
Galactose;
 N-acetylglucosamine;
N-acetylglucosamine;
 Mannose;
Mannose;
 Fucose.
Fucose.
 Sialic acid (N-acetylneuraminic acid);
Sialic acid (N-acetylneuraminic acid);
 Galactose;
Galactose;
 N-acetylglucosamine;
N-acetylglucosamine;
 Mannose;
Mannose;
 Fucose.
Fucose.| Peak No. | Peak ID | Structure |
|---|---|---|
| 1 | FA4BG4S4 |  |
| 2 | A2G2S2 |  |
| 3 | A2BG2S2; M3 |  |
| 4 | FA2G2S2 |  |
| 5 | FA2BG2S2 |  |
| 6 | FA2G1S1 |  |
| 7 | A2G2S1 |  |
| 8 | A2BG2S1 |  |
| 9 | FA2G2S1 |  |
| 10 | FA2BG2S1 |  |
| 11 | A4G4S2 |  |
| 12 | FA2; M6 |  |
| 13 | FA2[6]G1; M7 |  |
| 14 | FA2[3]G1; FA2BG1 |  |
| 15 | FA2G2 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torok, R.; Horompoly, K.; Szigeti, M.; Guttman, A.; Vitai, M.; Koranyi, L.; Jarvas, G. N-Glycosylation Profiling of Human Blood in Type 2 Diabetes by Capillary Electrophoresis: A Preliminary Study. Molecules 2021, 26, 6399. https://doi.org/10.3390/molecules26216399
Torok R, Horompoly K, Szigeti M, Guttman A, Vitai M, Koranyi L, Jarvas G. N-Glycosylation Profiling of Human Blood in Type 2 Diabetes by Capillary Electrophoresis: A Preliminary Study. Molecules. 2021; 26(21):6399. https://doi.org/10.3390/molecules26216399
Chicago/Turabian StyleTorok, Rebeka, Klaudia Horompoly, Marton Szigeti, Andras Guttman, Marta Vitai, Laszlo Koranyi, and Gabor Jarvas. 2021. "N-Glycosylation Profiling of Human Blood in Type 2 Diabetes by Capillary Electrophoresis: A Preliminary Study" Molecules 26, no. 21: 6399. https://doi.org/10.3390/molecules26216399
APA StyleTorok, R., Horompoly, K., Szigeti, M., Guttman, A., Vitai, M., Koranyi, L., & Jarvas, G. (2021). N-Glycosylation Profiling of Human Blood in Type 2 Diabetes by Capillary Electrophoresis: A Preliminary Study. Molecules, 26(21), 6399. https://doi.org/10.3390/molecules26216399