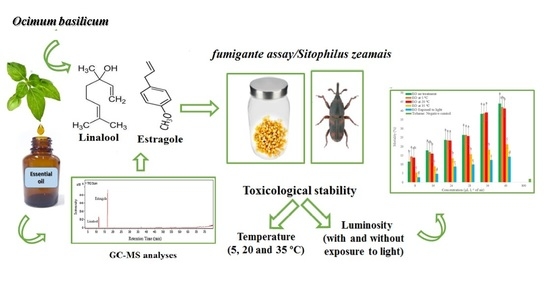
Toxicological Stability of Ocimum basilicum Essential Oil and Its Major Components in the Control of Sitophilus zeamais
Abstract
:1. Introduction
2. Material and Methods
2.1. Insect Colony
2.2. Essential Oil
2.3. Essential Oil Analysis
2.4. Exposure to Temperature and Light Radiation
2.5. Toxicological Stability
2.6. Statistical Analysis
3. Results and Discussion
3.1. Essential Oil Composition
3.2. Toxicological Stability of Essential Oil
3.3. Toxicological Stability of Linalool and Estragole
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Ahn, W.C.; Kalish, S.A.; Gelman, D.L.; Medin, C.; Luhmann, S.; Atran, J.D.; Coley, P.; Shafto, P. Why essences are essential in the psychology of concepts: Commentary on Strevens. Cognition 2001, 15, 237–262. [Google Scholar] [CrossRef]
- Franz, C.M.; Novak, J. Sources of essential oils. In Handbook of Essential Oils. Science, Technology, and Applications; Başer, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 39–81. [Google Scholar]
- Dornic, N.; Ficheux, A.S.; Roudot, A.C. Qualitative and quantitative composition of essential oils: A literature based database on contact allergens used for safety assessment. Regul. Toxicol. Pharm. 2016, 80, 226–232. [Google Scholar] [CrossRef]
- Oliveira, J.V.; França, S.M.; Barbosa, D.R.S.; Dutra, K.A.; Araújo, A.M.N.; Navarro, D.M.A.F. Fumigation and repellency of essential oils against Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae) in cowpea. Pesqui. Agropecu. Bras. 2017, 52, 10–17. [Google Scholar] [CrossRef]
- Correa, Y.D.C.G.; Faroni, L.R.A.; Haddi, K.; Oliveira, E.E.; Pereira, E.J.G. Locomotory and physiological responses induced by clove and cinnamon essential oils in the maize weevil Sitophilus zeamais. Pestic. Biochem. Phys. 2015, 125, 31–37. [Google Scholar] [CrossRef]
- Costa, C.M.G.R.; Santos, M.S.; Barros, H.M.M.; Agra, P.F.M.; Farias, M.A.A. Efeito inibitório do óleo essencial de manjericão sobre o crescimento in vitro de Erwinia carotovora. Rev. Tecnol. Ciên. Agropec. 2009, 3, 35–38. [Google Scholar]
- Nagaia, A.; Duarte, L.M.L.; Santos, D.Y.A.C. Influence of Viral Infection on Essential Oil Composition of Ocimum basilicum (Lamiaceae). Nat. Prod. Commun. 2011, 6, 1189–1192. [Google Scholar] [CrossRef] [Green Version]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Goswami, P.; Singh, V.R.; Verma, S.K.; Darokar, M.P.; Kurmi, A.; Singh, M.; Saikia, D.; et al. Essential Oil Composition and Antimicrobial Activity of Methyl cinnamate-Linalool Chemovariant of Ocimum basilicum L. from India. Rec. Nat. Prod. 2017, 11, 193–204. [Google Scholar]
- Mazzonetto, F.; Vendramim, J.D. Efeito de Pós de Origem Vegetal sobre Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) em Feijão Armazenado. Neotrop. Entomol. 2003, 32, 145–149. [Google Scholar] [CrossRef] [Green Version]
- López, M.D.; Jordán, M.J.; Pascual-Villalobos, M.J. Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. J. Stored Prod. Res. 2008, 44, 273–278. [Google Scholar] [CrossRef]
- Asawalam, E.F.; Emosairue, S.O.; Hassanali, A. Essential oil of Ocimum grattissimum (Labiatae) as Sitophilus zeamais (Coleoptera: Curculionidae) protectant. Afr. J. Biotechnol. 2008, 7, 3771–3776. [Google Scholar]
- Mishra, B.B.; Tripathi, S.P.; Tripathi, C.P.M. Repellent effect of leaves essential oils from Eucalyptus globulus (Mirtaceae) and Ocimum basilicum (Lamiaceae) against two major stored grain insect pests of Coleopterons. Nat. Sci. 2012, 10, 50–54. [Google Scholar]
- França, S.M.; Oliveira, J.V.; Esteves Filho, A.B.; Oliveira, C.M. Toxicity and repellency of essential oils to Zabrotes subfasciatus (Boheman) (Coleoptera, Chrysomelidae, Bruchinae) in Phaseolus vulgaris L. Acta Amaz. 2012, 42, 381–386. [Google Scholar] [CrossRef]
- Belong, P.; Ntonga, P.A.; Bakwo Fils, E.M.; Dadji, G.A.F.; Tamesse, J.L. Chemical composition and residue activities of Ocimum canum Sims and Ocimum basilicum L essential oils on adult female Anopheles funestus ss. J Anim. Plant Sci. 2013, 19, 2854–2863. [Google Scholar]
- Popovic, Z.; Kostic, M.; Popovic, S.; Skorcic, S. Bioactivities of Essential Oils from Basil and Sage to Sitophilus oryzae L. Biotechnol Biotec. Eq. 2015, 20, 36–40. [Google Scholar] [CrossRef]
- Almeida, F.A.C.; Almeida, S.A.; Santos, N.R.; Gomes, J.P.; Araújo, M.E.R. Efeito de extratos alcoólicos de plantas sobre o caruncho do feijão vigna (Callosobruchus maculatus). Rev. Bras Eng. Agrícola Ambient. 2005, 9, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Lopez, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Mughal, M.H. Linalool: A mechanistic treatise. J. Nut. Food Res. Technol. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Chapman, R.F.; Bernays, E.A.; Simpson, S.J. Attraction and repulsion of the aphid Cavariella aegopodii by plant odors. J. Chem. Ecol. 1981, 7, 881. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Hamraoui, A. Fumigant toxic activity and reproductive inhibition induced by monoterpenes on Acanthoscelides obtectus (Say) (Coleoptera), a bruchid of kidney bean (Phaseolus vulgaris L.). J. Stored Prod. Res. 1995, 31, 291–299. [Google Scholar] [CrossRef]
- Cheng, S.S.; Liu, J.Y.; Tsai, K.H.; Chen, W.J.; Chang, S.T. Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J. Agric. Food Chem. 2004, 52, 4395–4400. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, M.; Naik, S.N.; Tewaryb, D.K.; Mittalc, P.K.; Yadavc, S. Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. Vector-Borne Zoonot. 2007, 44, 198–204. [Google Scholar]
- Smith, R.L.; Adams, T.B.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Rogers, A.E.; et al. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances, methyl eugenol and estragole. Food Chem. Toxicol. 2002, 40, 851–870. [Google Scholar] [CrossRef]
- De Vicenzi, M.; Silano, M.; MaialettI, F.; Scazzocchio, B. Constituents of aromatic plants: II. Estragole. Fitoterapia 2000, 71, 725–729. [Google Scholar] [CrossRef]
- Morais, S.M.; Catunda-Junior, F.E.A.C.; Da Silva, A.R.A.; Neto, J.S.M.; Rondina, D.; Leal-Cardoso, J.H. Antioxidant activity of essential oils from Northeastern Brazilian Croton species. Quím. Nova 2006, 29, 907–910. [Google Scholar] [CrossRef] [Green Version]
- Lopez, M.D.; Pascual-Villalobos, M.J. Are monoterpenoids and phenylpropanoids efficient inhibitors of acetylcholinesterase from stored product insect strains? Flavour Fragr. J. 2015, 30, 108–112. [Google Scholar] [CrossRef]
- Wang, C.F. Components and insecticidal activity against the maize weevils of Zanthoxylum schinifolium fruits and leaves. Molecules 2011, 16, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Mbata, G.N.; Pascual-Villalobos, M.J.; Payton, M.E. Comparative mortality of diapausing and nondiapausing larvae of Plodia interpunctella (Lepidoptera: Pyralidae) exposed to monoterpenoids and low pressure. J. Econ. Entomol. 2012, 105, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.N. Evaluation of the toxicity of the essential oils of some common Chinese spices against Liposcelis bostrychophila. Food Control 2012, 26, 486–490. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, D.W. Toxicity of basil and orange essential oils and their components against two coleopteran stored products insect pests. J. Asia Pac. Entomol. 2014, 17, 13–17. [Google Scholar] [CrossRef]
- Treibs, W. Gewinnung atherischer Ole durch Destillation, Extraktion und Pressung. A. Einleitung. In Die atherischen Ole Band I, 4th ed.; Gildemeister, E., Hoffmann, F., Eds.; Akademie-Verlag: Berlin, Germany, 1956; pp. 307–309. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of on insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Souza, A.P.; Vendramim, J.D. Atividade inseticida de extratos aquosos de meliáceas sobre Bemisia tabaci (Genn) biótipo B. Neotrop. Entomol. 2001, 30, 133–137. [Google Scholar] [CrossRef]
- Don-Pedro, K.N. Investigation of single and joint fumigant insecticidal action of citruspeel oil components. Pestic. Sci. 1996, 46, 79–84. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, B.H.; Choi, W.S.; Park, B.S.; Kim, J.G.; CampbelL, B.C. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L). Pest Manag. Sci. 2001, 57, 548–553. [Google Scholar] [CrossRef]
- Arena, J.S.; Peschiutta, M.L.; Calvimonte, H.; Zygadlo, J.A. Fumigant and repellent activities of different essential oils alone and combined against the maize weevil (Sitophilus zeamais motschulsky). MOJ Biorg. Org. Chem. 2017, 1, 249–253. [Google Scholar]
- Liao, M.; Xiao, J.; Zhou, L.; Liu, Y.; Wu, X.; Hua, R.; Wang, G.; Cao, H. Insecticidal Activity of Melaleuca alternifolia Essential Oil and RNA-Seq Analysis of Sitophilus zeamais Transcriptome in Response to Oil Fumigation. PLoS ONE 2016, 9, e0167748. [Google Scholar] [CrossRef] [Green Version]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Badaway, M.E.I.; El-Arami, S.A.A. Fumigant and contact toxicities monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Maroto, M.C.; Pardo, E.A.; Castillo-Muñoz, N.; Díaz-Maroto, I.J.; Pérez-Coello, M.S. Effect of storage conditions on volatile composition of dried rosemary (Rosmarinus officinalis L.) leaves. Flavour Fragr. J. 2009, 24, 245–250. [Google Scholar] [CrossRef]
- Atkins, P.W. Kurzlehrbuch Physikalische Chemie, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Glasl, H. Uber die Haltbarkeit von Terpenoiden in Extrakten und Losungen mit unterschiedlichem Alkoholgehalt. Arch. Pharm. 1975, 308, 88–93. [Google Scholar] [CrossRef]
- Gopalakrishnan, N. Studies on the storage quality of CO2-extracted cardamom and clove bud oils. J. Agric. Food Chem. 1994, 42, 796–798. [Google Scholar] [CrossRef]
- Braun, M.; Franz, G. Quality criteria of bitter fennel oil in the German Pharmacopoeia. Pharm. Pharmacol. Lett. 1999, 9, 48–51. [Google Scholar]
- Turek, C.; Stintzing, F.C. Impact of different storage conditions on the quality of selected essential oils. Int. Food Res. J. 2012, 46, 341–353. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Brandares, M.F.T.; Dar Juan, B.B.; Vulelban, A.M.; Anzaldo, F.E. Stability studies of essential oils from some Philippine plants I. Zingiber Officinale Rosc. Philipp. J. Sci. 1987, 116, 1–11. [Google Scholar]
- Walallawita, W.K.U.S.; Bopitiya, D.; Sivakanthan, S.; Jayawardana, N.W.I.A.; Madhujith, T. Comparison of oxidative stability of sesame (Sesamum indicum), soybean (Glycine max) and mahua (mee) (Madhuca longifolia) oils against photo-oxidation and autoxidation. Procedia Food Sci. 2016, 6, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Misharina, T.A.; Polshkov, A.N.; Ruchkina, E.L.; Medvedeva, I.B. Changes in the composition of the essential oil of marjoram during storage. Appl. Biochem. Microbiol. 2003, 39, 311–316. [Google Scholar] [CrossRef]
- Fazolin, M.; Estrela, J.L.V.; Monteiro, A.F.M.; Silva, I.M.; Gomes, L.P.; Silva, M.S.F. Synergistic potential of dillapiole-rich essential oil with synthetic pyrethroid insecticides against fall armyworm. Ciênc. Rural 2016, 46, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.G.; Landim, T.N.; Silva, S.M.; Cunha, J.P.A.R.; Sampaio, M.V. Toxicity of Ocimum basilicum essential oil combined with thiamethoxam to cotton aphid. Rev. Bras. Cienc. Agrar. 2019, 14, e565. [Google Scholar] [CrossRef]
- Mcgraw, G.W.; Hemingway, R.W.; Ingram, L.L., Jr.; Canady, C.S.; Mcgraw, W.B. Thermal degradation of terpenes: Camphene, Δ3-carene, limonene, and α-terpinene. Environ. Sci. Technol. 1999, 33, 4029–4033. [Google Scholar]
- Ternes, W. Naturwissenschaftliche Grundlagen der Lebensmittelzubereitung, 3rd ed.; Behr’s Verlag: Hamburg, Germany, 2008. [Google Scholar]
- Turek, C.; Stintzing, F.C. Evaluation of selected quality parameters to monitor essential oil alteration during storage. J. Food Sci. 2011, 76, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.G.L.; Cardoso, M.G.; Zacaroni, L.M.; Lima, R.K. Influência da luz e da temperatura sobre a oxidação do óleo essencial de Capim-Limão (Cymbopogon citratus (D.C.) STAPF). Quim. Nova 2008, 31, 1476–1480. [Google Scholar] [CrossRef] [Green Version]
- Brodkorb, D.; Gottschall, M.; Marmulla, R.; Luddeke, F.; Harder, J. Linalool Dehydratase-Isomerase, a Bifunctional Enzyme in the Anaerobic Degradation of Monoterpenes. J. Biol. Chem. 2010, 285, 30436–30442. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Components | LC50 (FI 95%) (µL L−1 of Air) | LC95 (FI 95%) (µL L−1 of Air) | Inclination (±MSE 1) | χ2 (df) | p |
|---|---|---|---|---|---|
| Essential oil | 25.4 (23.1–28.6) | 178.4 (161.2–196.2) | 1.94 ± 0.37 | 0.14 (7) | 0.98 |
| EO at 5 °C | 25.7 (23.7–29.1) | 172.3 (154.3–187.2) | 1.99 ± 0.37 | 0.09 (7) | 0.99 |
| EO at 20 °C | 25.8 (24.1–30.6) | 151.3 (139.4–192.3) | 2.13 ± 0.38 | 0.85 (7) | 0.83 |
| Components | LC50 (FI 95%) (µL L−1 of Air) | LC95 (FI 95%) (µL L−1 of Air) | Inclination (±MSE 1) | χ2 (df) | p |
|---|---|---|---|---|---|
| Linalool | 34.6 (31.6–41.2) | 330.3 (269.8–463.6) | 1.67 ± 0.22 | 5.57 (5) | 0.34 |
| Linalool at 5 °C | 33.9 (30.7–42.1) | 339.3 (301.3–498.9) | 1.64 ± 0.22 | 6.07 (5) | 0.29 |
| Linalool at 20 °C | 39.4 (33.5–48.3) | 382.7 (365.2–529.8) | 1.51 ± 0.22 | 6.91 (5) | 0.22 |
| Linalool at 35 °C | 132.4 (110.3–198.7) | 495.6 (427.5–612.6) | 1.39 ± 0.19 | 1.56 (5) | 0.98 |
| Linalool exposed to light | 178.9 (152.4–215.6) | 534.2 (518.3–725.5) | 1.53 ± 0.23 | 1.84 (5) | 0.96 |
| Components | LC50 (FI 95%) (µL L−1 of Air) | LC95 (FI 95%) (µL L−1 of Air) | Inclination (±MSE 1) | χ2 (df) | p |
|---|---|---|---|---|---|
| Estragole | 38.1 (35.6–45.7) | 314.0 (298.3–465.6) | 1.79 ± 0.22 | 5.48 (5) | 0.35 |
| Estragole at 5 °C | 37.2 (34.3–43.8) | 305.4 (287.1–419.4) | 1.79 ± 0.22 | 6.08 (5) | 0.29 |
| Estragole at 20 °C | 41.0 (38.9–49.2) | 335.0 (303.5–490.2) | 1.80 ± 0.23 | 4.38 (5) | 0.49 |
| Estragole at 35 °C | 56.5 (47.5–63.4) | 395.2 (347.6–511.5) | 1.94 ± 0.25 | 3.44 (5) | 0.63 |
| Estragole exposed to light | 53.6 (45.9–61.3) | 362.1 (323.7–501.7) | 1.98 ± 0.17 | 6.96 (5) | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, E.d.S.; Faroni, L.R.D.; Heleno, F.F.; Rodrigues, A.A.Z. Toxicological Stability of Ocimum basilicum Essential Oil and Its Major Components in the Control of Sitophilus zeamais. Molecules 2021, 26, 6483. https://doi.org/10.3390/molecules26216483
Moura EdS, Faroni LRD, Heleno FF, Rodrigues AAZ. Toxicological Stability of Ocimum basilicum Essential Oil and Its Major Components in the Control of Sitophilus zeamais. Molecules. 2021; 26(21):6483. https://doi.org/10.3390/molecules26216483
Chicago/Turabian StyleMoura, Eridiane da Silva, Lêda Rita D’Antonino Faroni, Fernanda Fernandes Heleno, and Alessandra Aparecida Zinato Rodrigues. 2021. "Toxicological Stability of Ocimum basilicum Essential Oil and Its Major Components in the Control of Sitophilus zeamais" Molecules 26, no. 21: 6483. https://doi.org/10.3390/molecules26216483
APA StyleMoura, E. d. S., Faroni, L. R. D., Heleno, F. F., & Rodrigues, A. A. Z. (2021). Toxicological Stability of Ocimum basilicum Essential Oil and Its Major Components in the Control of Sitophilus zeamais. Molecules, 26(21), 6483. https://doi.org/10.3390/molecules26216483